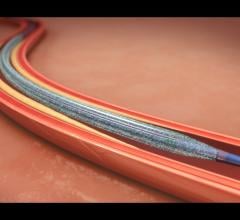
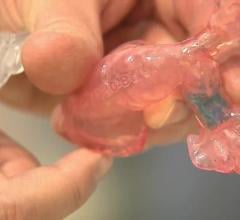
When a pediatric patient at Children’s Hospital Los Angeles needed a custom-build stent to repair his pulmonary artery ...
eHealth Technologies and HealtheConnections, a Syracuse-based regional health information organization (RHIO) supporting health information exchange (HIE) for the 11 counties of central and northern New York, have teamed up to share medical images across the care provider community.
February 9, 2017 — Janssen Research & Development LLC (Janssen) announced that the Phase 3 COMPASS trial is stopping ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Charleston Area Medical Center (CAMC) has documented reduced readmissions for congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD) and other chronic conditions. Driving this success is a comprehensive strategy supported by the SmarTigr interactive patient engagement and education system from TeleHealth Services.
Medtronic plc announced receipt of an investigational device exemption (IDE) from the U.S. Food and Drug Administration (FDA) to initiate a study of the IN.PACT Admiral drug-coated balloon (DCB) for a potential new indication in patients with end-stage renal disease. The randomized study will evaluate the IN.PACT Admiral DCB as a treatment for failing arteriovenous (AV) fistulas in these patients as compared to plain balloon angioplasty. The IDE approval enables Medtronic to initiate the study and gain safety and effectiveness data for the device in this investigational indication.
February 8, 2017 — Cardiovascular Systems (CSI) presented six-month data from its LIBERTY 360° post-market study in a ...
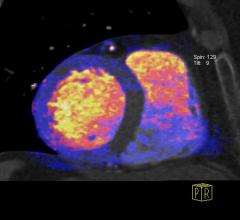
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
A new anticancer agent in development promotes regeneration of damaged heart muscle — an unexpected research finding that may help prevent congestive heart failure in the future.
GE Healthcare’s Life Sciences business announced in January that it acquired Rapidscan Pharma Solutions Inc., which has the exclusive rights to produce and sell the pharmacological stress agent Rapiscan (regadenoson) in territories outside the U.S., Canada and Mexico. GE Healthcare will help bring improved access to Rapiscan, offering an alternative screening method for patients who are unable to undergo traditional cardiac stress imaging procedures.
Healthcare analytics company Innovaccer Inc. announced the launch of its holistic MIPS Platform designed to enable providers to deliver better clinical outcomes. The platform does this by helping providers monitor performances, understand population, efficiently manage data and easily submit it to the Centers for Medicare and Medicaid Services (CMS).
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
February 7, 2017 — Of the more than 700,000 Americans who suffer a heart attack each year, about a quarter go on to ...
The supply chain can serve as a critical strategic asset when addressing important initiatives tied to managing costs ...
At the 2016 Radiological Society of North America (RSNA) annual meeting, GE Healthcare unveiled Freelium, a magnet technology designed to use 1 percent of liquid helium compared to conventional magnetic resonance imaging (MRI) magnets. Instead of the average 2,000 liters of precious liquid helium, Freelium is designed to use only about 20 liters.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
February 6, 2017 — At the Society of Cardiac Magnetic Resonance (SCMR) 20th Annual Scientific Sessions, GE Healthcare ...
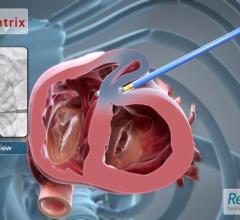
This video, provided by BioVentrix Inc., demonstrates how to implant the Revivent TC System to reduce the volume of the ...
Avinger Inc. recently announced positive two-year clinical data from the pivotal VISION study of the company’s Lumivascular technology. The VISION study was designed to evaluate the safety and effectiveness of Avinger’s Pantheris system to perform directional atherectomy while, for the first time ever, allowing physicians to use real-time intravascular imaging to aid in the removal of plaque from diseased lower extremity arteries. Data from the study, which demonstrated successful achievement of all primary and secondary safety and effectiveness endpoints, supported U.S. Food and Drug Administration (FDA) 510(k) clearance of the system in 2016.

 February 09, 2017
February 09, 2017