October 27, 2008 - Boston Scientific Corp. last week said the FDA approved its Carotid WALLSTENT Monorail Endoprosthesis for the treatment of patients with carotid artery disease who are at high risk for surgery.
The device is the leading carotid stent in Europe and other international markets, Boston Scientific said. The company said it plans to launch the product immediately in the U.S.
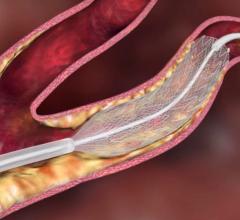
The Carotid WALLSTENT is a self-expanding stent mounted on a rapid exchange delivery system, designed to re-open the carotid artery by treating stenoses, and improve blood flow to the brain. The stent features a closed-cell design, engineered for excellent lesion coverage and angiographic results. The system is designed to be highly deliverable and provide access to the toughest lesions, the company said.
It is used in conjunction with the FilterWire EZ Embolic Protection System, which is designed to capture plaque debris released during the stenting procedure, preventing it from traveling to the brain, where it could create an increased risk for stroke. The device features filter sizing to accommodate vessel diameters between 3.5 mm and 5.5 mm.
“The closed-cell design of the Carotid WALLSTENT Endoprosthesis is intended to provide increased scaffolding for optimal lesion coverage and a smooth inner lumen,” said Barry T. Katzen, M.D., medical director, Baptist Cardiac and Vascular Institute, Miami. “This feature will make the Carotid WALLSTENT an attractive new treatment option for U.S. physicians and their patients.”
The Carotid WALLSTENT Endoprosthesis with the FilterWire EZ System is the only carotid artery stent system approved in the U.S. with an indication that includes the treatment of bilateral carotid artery disease (blockages in the carotid arteries on both sides of the neck).
Most patients with carotid artery disease are treated with carotid endarterectomy, a surgical procedure involving an incision in the neck and removal of the plaque from the vessel walls. Carotid artery stenting is a less-invasive alternative in which a stent is delivered to the site of the blockage and expanded, forcing open the walls of the arteries and restoring blood flow.
For more information: www.bostonscientific.com

 June 26, 2023
June 26, 2023