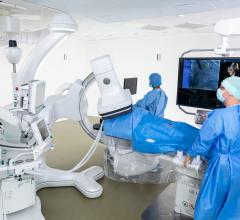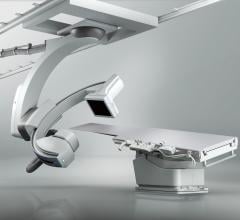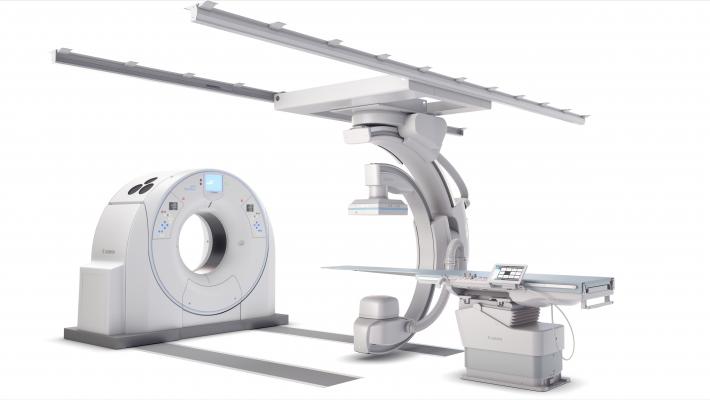
February 6, 2019 — Canon Medical Systems USA Inc. recently introduced a new angiography configuration featuring its Alphenix Sky + C-arm and Hybrid Catheterization Tilt/Cradle Table for interventional procedures with its Aquilion One/Genesis Edition computed tomography (CT) system. The new pairing, called the Alphenix 4-D CT, allows clinicians to efficiently plan, treat and verify in a single clinical setting. The flexible hybrid system enables streamlined workflow and extensive range of patient access and coverage.
Benefits include:
-
DoseRite technology designed to help clinicians minimize patient X-ray exposure while maintaining optimum image quality;
-
Outstanding patient access, enabling clinicians to move the system, not the patient, and utilizing the new tilting cradle table;
-
Greater control for the clinician with the tableside Alphenix Tablet to deliver a fast, seamless and rich workflow experience (optional);
-
Flexibility with C-arm flip, right or left lateral flexibility, speed and full-body 3-D imaging capability; and
-
Boosted productivity with the new Alphenix Workstation that integrates applications to help clinicians plan, analyze and perform interventional procedures
The new 4-D CT configuration is part of the new family of Alphenix angiography systems that delivers images with clarity and precision. The new family combines dose optimization technologies, enhanced workflow and innovative features to help clinicians provide patients with safe and fast treatment.
Canon Medical showcased its next-generation Alphenix platform at the 2018 Radiological Society of North America (RSNA) annual meeting, Nov. 25-30 in Chicago.
For more information: www.us.medical.canon


 January 31, 2024
January 31, 2024