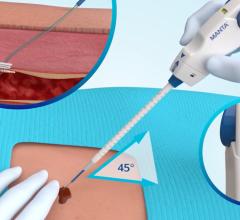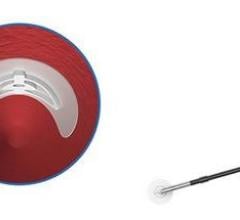
Abbott received FDA clearance to market its StarClose Vascular Closure System, a new vessel closure device engineered to enable fast, safe and secure closure and earlier patient mobilization after catheterization. StarClose introduces a tiny circumferential flexible clip onto the surface of the femoral artery, closing the artery securely in a matter of seconds following diagnostic catheterization procedures such as those used to diagnose coronary artery disease. The StarClose clip is designed for delivery "through-the-sheath" – a feature intended to avoid contact with the skin and thus decrease the risk of infection. Physicians can quickly and easily deliver the clip to the surface of a femoral artery with a series of four clicks of the device. The clip is made of nitinol, a nickel-titanium composite that “remembers” its shape once released from the StarClose device.


 April 16, 2024
April 16, 2024