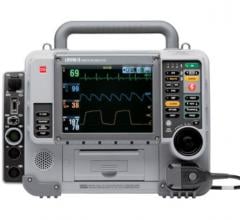
The “crash cart” defibrillator-monitor from Cardiac Science is designed specifically for use by medical professionals to respond to cardiac emergencies in hospital settings.
The new device is lightweight, rugged and portable, with resuscitation and pacing therapies designed for use by professionals in medically-supervised environments to respond to cardiac emergencies.
Operators are guided by a combination of programmable text prompts, audible alarms and visible indicators.
Incorporating STAR biphasic escalating shock energy technology, the defibrillator-monitor automatically adjusts the magnitude of the defibrillation shock based upon each patient’s unique body type and also employs RHYTHMx software, which provides heart rhythm analysis and shock advice to effectively address life-threatening arrhythmias. The product will be sold to hospitals in the U.S. and Canada under the Cardiac Science Powerheart brand, exclusively by GE Healthcare worldwide.

 July 28, 2023
July 28, 2023