Aug. 15, 2017 — The U.S, Food and Drug Administration (FDA) granted market clearance for the HAART 200 Aortic Annuloplasty Device, the first annuloplasty device designed specifically for bicuspid aortic valve repair. With FDA clearance of both the HAART 300 and HAART 200 Aortic Annuloplasty Devices, BioStable Science and Engineering Inc. is now able to offer U.S. surgeons a comprehensive portfolio of aortic valve repair solutions that addresses all forms of aortic valve insufficiency.
A Bicuspid aortic valve (BAV) is a congenital malformation where the aortic valve forms with only two functional valve leaflets instead of the normal three. BAV is the most common congenital heart defect, affecting up to 2 percent of the population, and carries significant risk of cardiovascular complications. Patients with BAV have significantly higher risk of developing aneurysms or dissections of the aorta and approximately 53 percent of patients will require aortic valve replacement within 25 years of being diagnosed.[1] Most BAV patients undergo aortic valve replacement between 40 and 60 years of age, subjecting them to increased risk of re-operation or complications associated with valve replacement technologies. Aortic valve repair is an emerging surgical alternative for patients with bicuspid aortic valve insufficiency that may offer improved patient outcomes compared to valve replacement.[2]
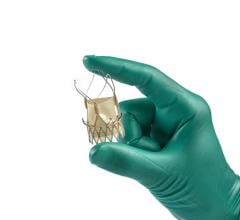
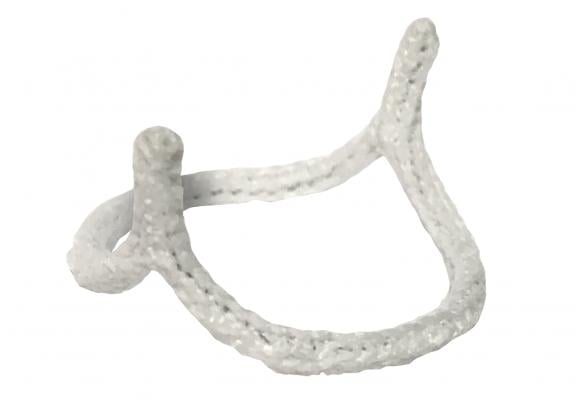
The HAART 200 Aortic Annuloplasty Device is designed to facilitate valve repair in patients with aortic valve insufficiency due to BAV. The device is designed to reduce annular diameter based upon leaflet size, to conform the annulus to a circular, symmetric shape for improved valve function, and to stabilize the annular geometry long-term.
“Surgical repair of the bicuspid aortic valve can be a complex three dimensional problem because of variability in the valve anatomy,” explained Scott Rankin, M.D., professor of surgery at West Virginia University Heart and Vascular Institute, and inventor of the HAART device. “Implantation of the HAART 200 Aortic Annuloplasty Device conforms the native valve to the three dimensional shape of the device, creating a circular valve geometry and aligning the leaflet commissures into the preferred 180 degree orientation. Conforming the native valve to this shape simplifies assessment and reconstruction of the valve leaflets and creates a central flow pattern of blood through the valve which may improve the long-term durability of the repair.”
The HAART 200 Aortic Annuloplasty Device is not available for sale outside of the United States.
For more information: www.biostable-s-e.com
Read the article "HAART 300 Aortic Annuloplasty Device Sees U.S. Pilot Launch, First Commercial Use."
Read the related article "Transcatheter Annuloplasty For Repair Versus Replacement in Functional Mitral Regurgitation."
References:


 March 20, 2024
March 20, 2024