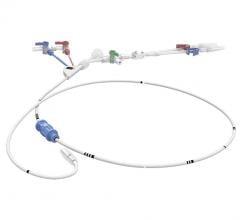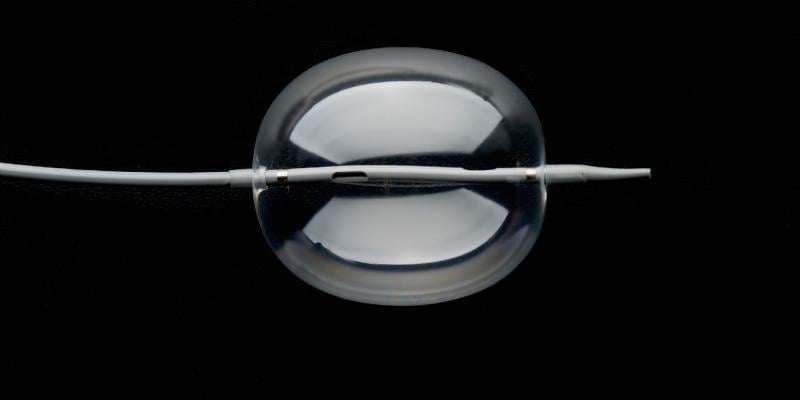
August 23, 2018 — W. L. Gore & Associates Inc. (Gore) announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the Gore Molding & Occlusion Balloon. The compliant polyurethane balloon catheter is designed to assist in the expansion of self-expanding stent grafts or to temporarily occlude large-diameter vessels. The device also received approval from the Japanese Ministry of Health, Labour and Welfare, and receipt of CE Mark. It meets all endovascular aortic repair (EVAR) procedural requirements, according to Gore – a single balloon that replaces the need for multiple molding and occlusion balloons.
The device’s radial expansion force across the range of EVAR device sizes enables physicians to consistently seat and seal grafts with confidence. This more efficient graft seal may reduce procedural time and the risk of Type 1 endoleaks. The device is also engineered with the lowest profile to reduce the potential of access-related complications, and its pushability and trackability offers enhanced control with uncompromised inflation and deflation time, according to Gore.
The new device is supplied in a single catheter length of 90 cm. This will enable use with current Gore Excluder Devices as well as future Gore devices while still being compatible with a 180 cm length guidewire.
Besides adding value through consistently reliable technical success and patient experience, the use of a single balloon allows for efficiency and inventory optimization. Use of a single molding and occlusion balloon during an EVAR case reduces intraoperative waste, minimizing overall instrument cost per procedure. The potential to reduce operating room and catheter lab time may contribute to faster room turnover and more on-time procedural starts, according to Gore.
For more information: www.goremedical.com


 April 04, 2024
April 04, 2024