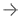
Abbott Espirit BTK Bioresorbable stent, or bioresorbable vascular scaffold (BVS) measures 99 microns and is made from poly-L-lactide (PLLA), a semi-crystalline bioresorbable polymer engineered to resist vessel recoil and provide a platform for drug delivery.
September 3, 2020 — Abbott today announced the start of the LIFE-BTK clinical trial to evaluate the safety and effectiveness of the company's new Esprit BTK Everolimus Eluting Resorbable Scaffold System. This is the first Investigational Device Exemption (IDE) trial in the U.S. to evaluate a fully bioresorbable stent to treat blocked arteries below the knees, or critical limb ischemia (CLI), in people battling advanced stages of peripheral artery disease (PAD). The first patient was enrolled by Danielle Bajakian, M.D., a vascular surgeon at New York-Presbyterian/Columbia University Irving Medical Center.
"Far too many people are impacted by peripheral artery disease, and this new drug-eluting resorbable scaffold is needed to offer meaningful improvements in how this disease is treated," said Nick West, M.D., divisional vice president, medical affairs, and chief medical officer in Abbott's vascular business. "Patients treated with balloon angioplasty often require repeat procedures on treated arteries, and therefore a drug-eluting resorbable device is ideally-suited to provide mechanical support for the vessel, reduce the chance of vessel re-narrowing and then gradually disappear over time."
For people with CLI, blocked vessels impair blood flow to the lower extremities, which can lead to severe pain, wounds, and in some cases, limb amputation. Globally, more than 200 million1 people suffer from PAD, disproportionally affecting people in underserved communities. Worse, up to 40% of patients with CLI currently undergo amputations,2 many of which could be prevented by eliminating health care disparities.
Currently, the standard of care for patients battling CLI is balloon angioplasty, which relies on a small balloon delivered via a catheter to the blockage to compress it against the arterial wall, opening the vessel and restoring blood flow. However, blockages treated only with balloon angioplasty have poor short- and long-term results, and in many instances the vessels become blocked again, requiring additional treatment.
There are no drug-eluting stents (DES), drug-coated balloons (DCB) or bare-metal stents (BMS) approved for use below the knee (BTK) in the U.S. With the limited options for BTK, new treatment options are needed. The U.S. Food and Drug Administration (FDA) has granted Esprit BTK breakthrough device designation, which streamlines review and pre-market approval timelines.

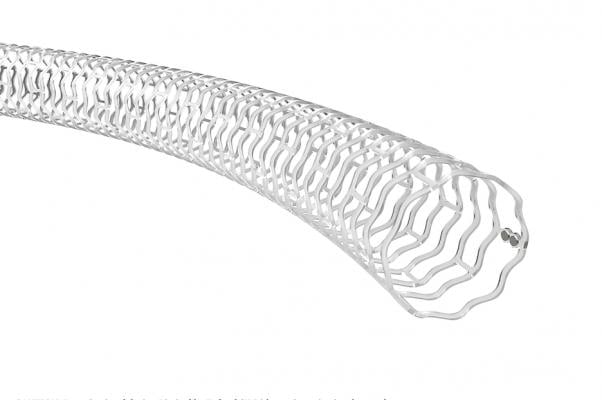
The Abbott Espirit BTK bioresorbable stent in the balloon expanded position.
Unlike traditional metal stents, Abbott's Esprit BTK System is not a permanent implant; it provides support to an artery immediately after a balloon angioplasty, preventing the vessel from reclosing. Once implanted, the scaffold delivers a drug over a few months that promotes healing and keeps the artery open. The scaffold is naturally resorbed into the body over time, like dissolving sutures, and ultimately leaves only a healed artery behind. Abbott is a pioneer in bioresorbable scaffolds. Long-term clinical data from a meta-analysis of randomized Absorb trials suggested that bioresorbable scaffolds might be an acceptable alternative to metallic DES for many patients with coronary artery disease.3
"After a five-year feasibility study in assessing treatment of arteries below the knee with a different version of this technology, the outcomes suggest resorbable devices have significant potential to become the favored therapy for CLI patients," said Ramon Varcoe, MBBS MS FRACS Ph.D., University New South Wales, Sydney, Australia, and one of the principal investigators in the trial. "This technology offers the best of both worlds. The device provides a strong scaffold structure and delivers anti-proliferative drugs, then disappears and is not an impediment for future interventions, surgery or imaging."
Once fully enrolled, the LIFE-BTK trial will evaluate the Esprit BTK resorbable scaffold in 225 patients at centers around the world. The study is the first of its kind and an evolution of prior studies examining resorbable technology in treating diseased vessels and blocked arteries.
About the LIFE-BTK Trial
The LIFE-BTK trial is the first IDE trial in the U.S. to evaluate a fully dissolvable device to treat critical limb ischemia in people battling advanced stages of peripheral artery disease (PAD). To date, the trial has sites in Australia, Japan, New Zealand, Singapore and the United States.
The LIFE-BTK trial is a prospective, randomized controlled clinical trial comparing Esprit BTK to Percutaneous Transluminal Angioplasty (PTA). The study objective is to evaluate the safety and efficacy in CLI patients with up to two lesions in separate infrapopliteal vessels, one of three types of vessels found below the knee and the type most commonly affected by CLI.
The trial will be led by principal investigators Brian DeRubertis, M.D. (vascular surgeon, UCLA), Sahil Parikh M.D., (interventional cardiologist, New York-Presbyterian/Columbia University Irving Medical Center, who serves on the medical advisory board for Abbott's vascular division), and Ramon Varcoe MBBS MS FRACS Ph.D., (University New South Wales, Sydney, Australia).
Renewed Interest in Bioresorbable Stents 3 Years After ABSORB Was Pulled From the Market
There was growing enthusiasm for bioresorbable scaffold technologies as the next step in stent evolution 5-6 years ago and led to the FDA clearance the fully resorbable stent in 2015, the Abbott Absorb bioresorbable vascular scaffold (BVS). However, trial data from the ABSORB III Trial led to first commercial bioresorbable stent being pulled off the market on Sept. 14, 2017. This led to questions about the future of the technology.
Watch the VIDEO: What Went Wrong With the Absorb Stent? — interview with Ajay Kirtane, M.D.
The Absorb device was not selling well, but data from several ABSORB trials statistically showed good performance compared to the market-leading Xience everolimus-eluting metallic stent. However, there was signal in the data for slightly poorer outcomes, negating any long-term benefits the stent might offer. Experts involved in the trials said Absorb saw a very low usage rate, with estimates of U.S, usage of less than 5 percent. This lead to other vendors like Boston Scientific to shelve its own BVS research. In 2018, Abbott said it would continue BVS research and hoped to launch future trials for an improved device in niche applications, which is what is now being done with the Esprit BTK.
About the Esprit BTK Bioresorbable Stent System
The Esprit BTK System consists of a thin strutted scaffold which measures 99 microns made from poly-L-lactide (PLLA), a semi-crystalline bioresorbable polymer engineered to resist vessel recoil and provide a platform for drug delivery. The scaffold is uniformly coated with poly-D, L-lactide (PDLLA) and the cytostatic drug, everolimus. PDLLA is an amorphous bioresorbable polymer coating designed to allow controlled drug release. The controlled release of everolimus, a drug that acts to retard cell proliferation, reduces scar tissue growth in the affected area, which can cause vessel blockage. The Esprit BTK System is an investigational device only in the U.S.
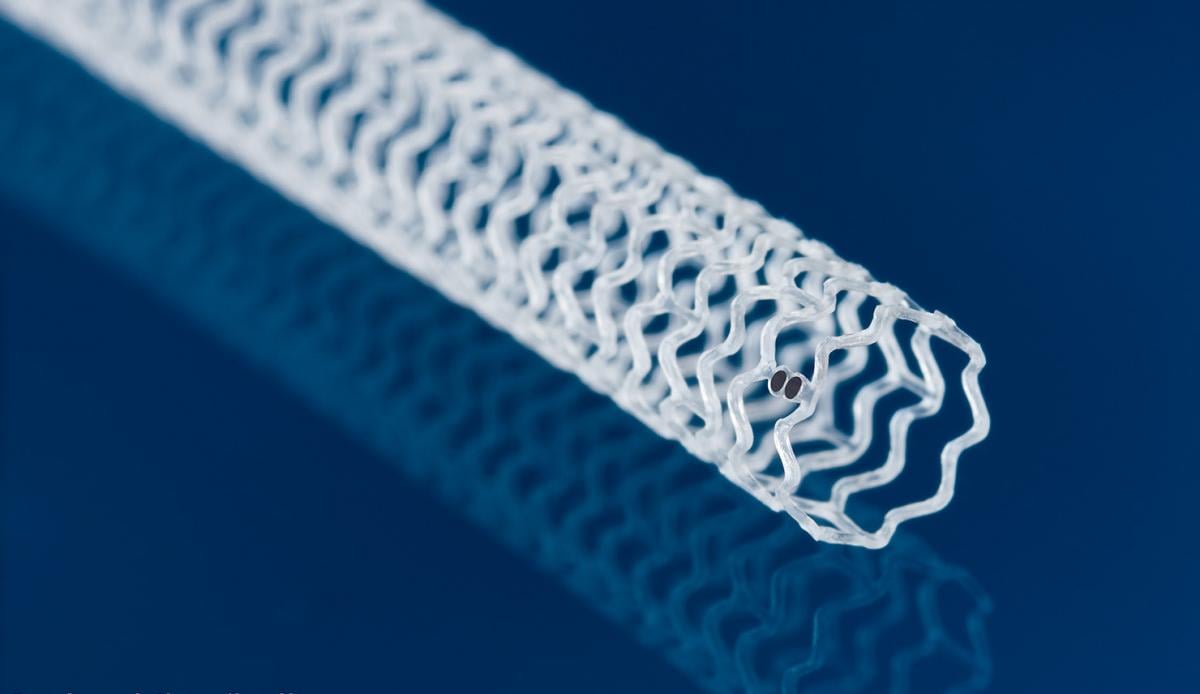
Abbott Espirit BTK Bioresorbable stent, or bioresorbable vascular scaffold (BVS) measures 99 microns and is made from poly-L-lactide (PLLA), a semi-crystalline bioresorbable polymer engineered to resist vessel recoil and provide a platform for drug delivery.
About Critical Limb Ischemia
Critical limb ischemia is a severe form of PAD that is characterized by chronic pain, even at rest, as well as ulcers and gangrene (tissue death) that develop as a result of chronically poor blood flow to the lower limbs. The most common endovascular treatment of below-the-knee arteries is balloon angioplasty, which opens narrowed arteries with a catheter-guided balloon. For some patients, surgical bypass procedures are performed. If left untreated, the disease can eventually lead to amputation and limb loss.
Related Resorbable Stent Content:
Current State of Bioresorbable Stent Technology — details the issues raised in the ABSORB III Trial presented at TCT 2017
VIDEO: The Current State of Bioresorbable Stents in 2018 — Interview with Patrick Serruys, M.D.
Bioresorbable Stent Shows Good 5-Year Outcomes in ABSORB BTK Trial
The Next Step in Bioresorbable Stent Technologies
One-Year Results of the DISAPEAR Registry Positive for Absorb Stent
VIDEO: Poor Outcomes for Bioresorbable Stents in Small Coronary Arteries — interview with Gregg Stone, M.D., at ACC 2016
Abbott Will End Sales of Absorb Bioresorbable Stent
VIDEO: What Went Wrong With the Absorb Stent? — interview with Ajay Kirtane, M.D., at TCT 2017
VIDEO: Bioresorbable Stent Comparable to Xience at Two Years, With Concerns — interview with Stephen Ellis, M.D., at ACC 2017
Tempering the Bioresorbable Stent Euphoria Following FDA Clearance of the Absorb
VIDEO: Bioresorbable Stent Failure Modes, Technique, Patient Selection and Future Development — a discussion with Juan Granada, M.D.
Bioresorbable Stent Comparable to Xience at Two Year, But With Adverse Trends
VIDEO: Editor’s Choice of the Most Innovative New Technology at TCT 2018
VIDEO: Future Research and Development Efforts in Cardiovascular Medicine — Interview with Professor Ian Meredith, MBBS
References:
1. Fowkes, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: 1329-40
2. https://www.amputee-coalition.org/resources/limb-loss-statistics/
3. Stone, et al. Time-Varying Outcomes With the Absorb Bioresorbable Vascular Scaffold During 5-Year Follow-up: A Systematic Meta-analysis and Individual Patient Data Pooled StudyJAMA Cardiol 2019 Dec 1;4(12):1261-1269. doi: 10.1001/jamacardio.2019.4101.


 January 05, 2026
January 05, 2026