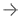The Acutus Medical AcQBlate Force sensing ablation catheter.
April 5, 2021 — Acutus Medical today announced initial U.S. enrollments in the company’s AcQForce Flutter Investigational Device Exemption (IDE) clinical trial. This trial is expected to enroll up to 150 subjects in leading centers globally and will evaluate the safety and efficacy of the AcQBlate Force sensing ablation catheter and system in the treatment of right atrial typical flutter.
In the U.S., right atrial typical flutter ablation procedures currently account for approximately 30% of cardiac ablations and are expected to reach 200,000 annually by 2025.[1]
In contrast to the most contemporary ablation systems, AcQBlate Force can operate both in a stand-alone manner or in conjunction with a compatible 3-D mapping system. The AcQBlate Force sensing ablation system is comprised of Acutus’ AcQBlate Force catheter and Qubic Force module that seamlessly integrates an RF generator and irrigation pump. The complete AcQBlate Force sensing ablation catheter and system, which received full European CE mark in late 2020, is now commercially available in Europe through our Acutus direct organization and our partner Biotronik.
Designed specifically to provide consistent, effective therapeutic solutions during cardiac ablation procedures, the AcQBlate Force system shows physicians, in real-time, how much contact force is being applied to the heart during ablations. Studies have shown the utility of real-time contact force information in helping physicians guide safe and effective therapy, which may improve patient outcomes.[2]
“The AcQBlate Force sensing ablation catheter provides stable contact force readings with low fluid irrigation requirements. It displayed both easy maneuverability and excellent stability during atrial ablation. I look forward to further evaluating this system and believe this technology has the potential to improve patient outcomes,” said Gery Tomassoni, M.D., an electrophysiologist at Baptist Health Medical Group in Lexington, Ky., who performed the first case in the trial.
“The system was very easy for our lab staff to set up, and we were able to complete the case without changing our existing workflow," explained Sean Beinart, M.D., FACC, FHRS, an electrophysiologist at Adventist Healthcare White Oak Medical Center in Silver Springs, Md. As the incidence of atrial arrhythmias increases, we continue to look for safe, effective and efficient treatment options for patients. The AcQBlate Force sensing ablation catheter incorporates a gold-tipped electrode that is designed for efficient energy delivery and effective lesion creation in less time.”
“This trial represents our initial entry into the United States with a therapeutic technology for the treatment of atrial arrhythmias,” said Vince Burgess, CEO of Acutus Medical. “The gold-tipped AcQBlate Force catheter is a state-of-the-art ablation device that is paired with our laser-based contact force console and further integrated with the smallest and most modern RF generator and pump available. As we have experienced in our European centers during our initial market release, when used in concert with our novel AcQMap 3-D mapping system we are seeing highly efficient workflow and operational efficiency. We look forward to offering the benefits of a comprehensive, force sensing ablation system to electrophysiologists and patients in the United States once we complete clinical trials and gain regulatory approval.”
Enrollment for the AcQForce Flutter trial is ongoing, and the company is actively working with selected sites to initiate cases under the IDE. For more information on this trial. AcQBlate Force Sensing Catheter is limited by U.S. federal law to investigational use.
Related EP Ablation Technology Content:
VIDEO: Early Ablation Improved Outcomes in Atrial Fibrillation Patients —interview with Oussama Wazni, M.D.
Esophageal Cooling May Help Prevent Injury From Cardiac Ablations
VIDEO: Top New EP Technologies at Heart Rhythm Society 2020 — Interview with Andrew Krahn, M.D.
Biotronik Partners With Acutus Medical to Offer More Efficient Arrhythmia Diagnosis and Treatment
Contact Force Sensing Catheter Improved Outcomes in Persistent Atrial Fibrillation Ablation
New Technologies to Improve Atrial Fibrillation Ablation
VIDEO: Current State of Atrial Fibrillation Ablation Technologies, an interview with Hugh Calkins, M.D., at HRS 2017.
Find more EP technology news and video


 February 06, 2026
February 06, 2026