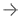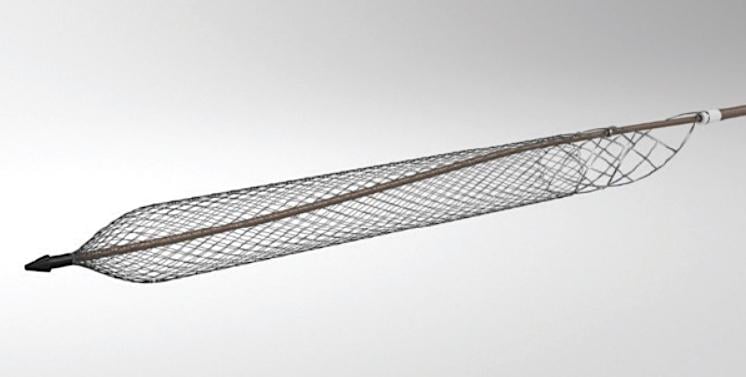
The ClotTriever system (Inari Medical), a mechanical thrombectomy system FDA 510(k) cleared for the nonsurgical removal of soft thrombi and emboli from peripheral blood vessels.
November 7, 2019 — The ClotTriever Outcomes (CLOUT) Registry is evaluating real-world patient outcomes following treatment of acute and non-acute lower extremity proximal deep vein thrombosis (DVT) with the ClotTriever system (Inari Medical), a mechanical thrombectomy system FDA 510(k) cleared for the nonsurgical removal of soft thrombi and emboli from peripheral blood vessels. The most recent data were presented at the 2019 Vascular Interventional Advances (VIVA) annual meeting.
The CLOUT Registry is enrolling 500 patients with lower extremity DVT involving the femoral vein, iliac vein, and/or inferior vena cava at up to 50 sites. Thrombus age is not capped, including acute, subacute, and chronic clot. Patients with vena cava filters and venous stents are excluded. Baseline demographics, comorbidities, eligibility for thrombolytic treatment, and DVT-specific baseline characteristics are collected prior to the ClotTriever procedure. Patient outcomes are collected at hospital discharge following thrombectomy and at 30 days, 6 months, 1 year, and 2 years post-procedure.
Procedural and acute data from 50 patients across 12 sites and 30-day follow-up data of 37 patients were assessed. Of these, 98% of patients were treated in a single session with a median thrombectomy time of 38 minutes, explained presenter David Dexter, M.D., Sentara Vascular Specialists.
Thrombus removal of ≥ 75% via core lab–assessed Marder scores was achieved in 76.5% of treated limbs, including those with chronic disease. There was one (2%) major adverse event, neither procedure nor DVT related: a death 23 days post-procedure due to sepsis and renal failure in a metastatic lung cancer patient. No bleeding complications and one (2%) access site hematoma was reported. At 30-day follow-up, the number of patients with post-thrombotic syndrome (PTS) as well as moderate or severe PTS had significantly decreased (P<0.01). Additionally, quality of life scores, including Villalta, revised Venous Clinical Severity Score, EQ-5D, and Numeric Pain Rating Scale, showed statistically significant improvement at 30 days. Further registry enrollment and analysis will provide insight for future definitive studies for the treatment of DVT.
Find information on all the VIVA 2019 Late-breaking Clinical Trials


 January 05, 2026
January 05, 2026