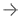July 22, 2020 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific's next generation Watchman FLX Left Atrial Appendage Closure (LAAC) Device.
The Watchman FLX is indicated to reduce the risk of stroke in patients with non-valvular atrial fibrillation (NVAF) who need an alternative to oral anticoagulation therapy by permanently closing off the left atrial appendage – the area of the heart where stroke-causing blood clots commonly form in NVAF.
The redesigned device is built on the most studied and implanted LAA occluder device in the world. It features a new, fully rounded design that offers physicians the ability to safely enter, and maneuver within, the left atrial appendage. It is the first LAA occluder that can be fully recaptured, repositioned and redeployed for precise placement, and the new frame design allows for optimal device engagement with the tissue for long-term stability and a faster, more complete seal. The Watchman FLX device is available in broader size options than the previous generation device and can treat a wider range of patient anatomies.
"The device sizes have much more overlap among each other, which allows for higher compression ratio by 10-30 percent, which allows me to choose among the devices in a more precise manner. That helps with choosing the right device for the anatomy rather than trying to fit a device into the appendage," explained Devi Nair, M.D., an electrophysiologist at St. Barnards Medical Center, Jonesboro, Ark.
 Positive 12-month results from the pivotal PINNACLE FLX study, which evaluated the performance of the Watchman FLX device as an alternative to long-term non-vitamin K antagonist oral anticoagulants (NOACs) and other OAC medications, were recently presented as a late-breaking clinical trial at Heart Rhythm Society 2020 Science. The study met its primary safety and efficacy endpoints with data demonstrating a low rate of major procedure-related safety events (0.5% at 7 days post procedure) and high rate of effective LAAC (100% with peri-device flow < 5mm at 12 months post procedure). Data also demonstrated a high implant success rate of 98.8%.
Positive 12-month results from the pivotal PINNACLE FLX study, which evaluated the performance of the Watchman FLX device as an alternative to long-term non-vitamin K antagonist oral anticoagulants (NOACs) and other OAC medications, were recently presented as a late-breaking clinical trial at Heart Rhythm Society 2020 Science. The study met its primary safety and efficacy endpoints with data demonstrating a low rate of major procedure-related safety events (0.5% at 7 days post procedure) and high rate of effective LAAC (100% with peri-device flow < 5mm at 12 months post procedure). Data also demonstrated a high implant success rate of 98.8%.
"Built upon the success of the Watchman platform and thousands of patient-years of clinical research, the next-generation Watchman FLX device is designed to offer increased ease of use for physicians and improved procedural outcomes for patients, including reduced complication risk and healing time," said Dr. Ian Meredith, AM, global chief medical officer, Boston Scientific. "We've set the bar high and look forward to bringing these benefits to a wider range of U.S. patients with NVAF who need an alternative to the bleeding risk and lifestyle challenges associated with long-term use of blood thinners."
Additional clinical research using the Watchman FLX device for patients with NVAF will continue via enrollment in the OPTION trial – comparing the device to oral anticoagulants in patients who also undergo a cardiac ablation procedure – as well as in the CHAMPION-AF trial, which will study a broader OAC-eligible patient population in a head-to-head fashion to compare the device against NOACs.
"We've been very pleased with the real-world clinical outcomes and positive physician feedback for the Watchman FLX device in Europe and are excited to extend availability of this next-generation technology to patients and clinicians throughout the U.S.," said Joe Fitzgerald, president, interventional cardiology, Boston Scientific. "Our Watchman technology was the first FDA-approved LAAC device on the market and has been implanted in more than 100,000 patients worldwide, and now with the Watchman FLX device, we are taking the clinical benefits of the technology to the next level for more patients while further differentiating our structural heart portfolio in the U.S."
The company announced CE mark for the next-generation Watchman FLX device in March 2019 and will immediately commence a limited launch of the device in the U.S.
For more information: www.watchman.com/implanter
See an overview and demonstration video of the new LAA occluder: https://www.multivu.com/players/English/8707351-boston-scientific-watchman-flx-device-fda-approved/
Related Content on the Watchman FLX LAA Occluder:
Boston Scientific Receives CE Mark For Next-generation Watchman FLX LAA Closure Device
Occluding the Left Atrial Appendage (LAA)
Update for LAA Occluder Technology News and Trends
PINNACLE FLX Study of New Watchman FLX LAA Occluder Meets Safety and Efficacy Endpoints
Watchman FLX in Clinical Trial at MedStar Heart and Vascular Institute
Boston Scientific Initiates OPTION Trial of Watchman FLX


 December 19, 2025
December 19, 2025