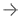
An example of the Medis QCA angiography imaging derived fractional flow reserve (FFR) assessment. This technology removes the need for pressure wires and adenosine used in traditional FFR assessments of the coronary arteries.
July 22, 2021 — Medis Medical Imaging is partnering with CORRIB Core Lab and Sinomed in randomized clinical trial of angiography-derived fractional flow reserve (FFR) using the Medis Quantitative Flow Ratio (QFR) technology. It uses imaging data from a rational angiography in the cath lab to determine the amount of blood flow through a vascular lesion to determine if it needs to be stetted, or if it can be treated medically.
The PIONEER IV study is a randomized, multicenter trial comparing clinical outcomes between angiography-derived physiology guidance by QFR and local routine diagnostic procedure and usual care in an all-comers patient population undergoing percutaneous coronary intervention (PCI) with an unrestricted use of Sinomed’s new drug-eluting stent, the Healing-Targeted Supreme Stent (HT Supreme).
The PIONEER-IV study will involve careful physiological identification of heart blood vessels that need to be treated or deferred. A narrowing located in a coronary vessel is deemed flow limiting if QFR <0.80. After stenting, the QFR measurement is repeated in the stented vessel(s) to verify the success of the intervention. In case distal QFR is <0.91 or delta QFR (across the stent is >0.05), post-dilatation of stented segment or additional stenting is recommended.
The PIONEER-IV trial will take place in 30 hospital centers across Europe and involve 2,540 coronary artery disease patients.
The trial is sponsored by NUI Galway and it will be centrally coordinated by the University’s Corrib Research Centre for Advanced Imaging and Core Laboratory.
This Pioneer IV trial will be executed with the QFR-software of Medis Medical Imaging as the image-based solution for the physiologic assessment of the severity of coronary lesions.
The trial is co-chaired by NUI Galway’s CORRIB Core Lab, led by Professor Patrick W. Serruys, Established Professor of Interventional Medicine and Innovation, and Professor William Wijns, Science Foundation Ireland Professor of Interventional Cardiology, both of whom are internationally renowned experts in interventional cardiology and cardiovascular disease.
“This trial is of paramount importance and is a critical attempt at replacing pressure-wire guidance by a physiological guidance derived from conventional coronary angiography using the on-line QFR software of Medis Medical Imaging," explained Serruys. "The cost-effectiveness of this approach is obvious and may signify a turning point in the physiological guidance of PCI. Furthermore, the trial is an all-comers trial, applying novel P2Y12 monotherapy and using a stent specially designed for fast and healthy endothelial coverage of the struts — the Healing Targeted Supreme DES."
The PIONEER IV trial is unique in randomizing all-comer patients, said Professor Osama Soliman, medical director of the CORRIB corelab. "This trial may pave a way to facilitate the adoption of physiology-guided stenting by performing on-line QFR in all patients treated in a cath lab. The standard use of HT Supreme DES in the PIONEER IV trial will promote the short-DAPT strategy rationalized by its excellent early tissue coverage as demonstrated on optical coherence tomography in the PIONEER-II study, and eliminate any potential bias caused by the selection of different DES type," he said.
PIONEER-IV is the first trial exploring the use of image-based physiology for both planning and optimization of percutaneous coronary interventions. As this strategy will be tested in an unselected, all-comers patient population, the results of PIONEER IV will complement those of ongoing trials like FAVOR III comparing QFR and FFR in more selected patients.
"We are pleased that CORRIB Core Lab has chosen QFR as the image-based solution for the physiologic assessment of the severity of the coronary lesions in this Pioneer IV Trial," said Professor Johan H.C. Reiber, chief scientific officer at Medis Medical Imaging. "The QFR has proven its robustness and quality in more than 15,000 patients collected in European and Asian countries and the results have been published in more than 105 peer-reviewed scientific publications."
For more information: www.medisimaging.com, www.nuigalway.ie/corrib-corelab/, www.sinomed.com
Related Content:
VIDEO: Example of the Medis QFR Angiography Image Derived FFR System
Biodegradable HT Supreme Drug Eluting Coronary Stent Noninferior to Xience or Promus Durable Polymer Stents
Medis QAngio XA 3D Receives FDA 510k Clearance
Novel FFR Methods Can Reduce Procedure Time and Cost
Image-based FFR May Replace Pressure Wires and Adenosine
CathWorks FFRangio System Receives U.S. FDA Clearance
Find more news and new technology in the FFR Technology Channel


 January 05, 2026
January 05, 2026