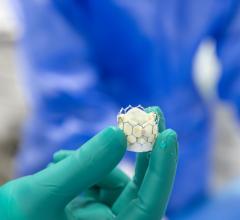
May 10, 2016 — Medtronic plc announced clinical results highlighting the strong safety and performance profile of the miniaturized Micra Transcatheter Pacing System (TPS) at Heart Rhythm 2016, the Heart Rhythm Society's 37th Annual Scientific Sessions, May 4-7 in San Francisco.
The Micra TPS is less than one-tenth the size of traditional pacemakers, and Medtronic claims it is the only leadless pacemaker approved for use in both the United States and Europe. Data presented at HRS further underscored the technology’s safety and performance profile. The data also confirmed Micra’s ability to accurately respond to patients’ activity levels by adjusting therapy using, for the first time, an accelerometer sensor positioned within the heart.
“Many patients with bradycardia require rate-responsive pacing so that their heart rates increase during exercise,” said Razali Omar, M.D., senior consultant cardiologist at the National Heart Institute in Kuala Lumpur, Malaysia. “Conventional pacemakers use various sensors outside the heart to detect patient activity, but even these sensors can have difficulty detecting moderate physical activity. As data presented today show, the Micra accurately responds to patients' activity levels by adjusting therapy when needed using a sensor within the heart.”
Using a miniaturized sensor to measure a patient's movement (also known as an accelerometer), the Micra TPS determines which pacing rates are appropriate based on patient activity levels. Medtronic said the technology is the first U.S. Food and Drug Administration (FDA)-approved cardiac device to position the sensor inside the heart and the only leadless pacemaker to offer the benefits of accelerometer-based sensing technology, the industry standard for traditional pacemaker systems.
Data presented at HRS assessed the rate response performance for approximately 20 patients with Micra TPS at three and six months post-implant procedure. In the study, patients underwent treadmill tests exercising to maximal exertion. The study found that appropriate rate-responsive pacing is achievable with an entirely intracardiac accelerometer-based pacing system.
Comparable in size to a large vitamin, the Micra TPS is attached to the heart with small tines and delivers electrical impulses that pace the heart through an electrode at the end of the device. Unlike traditional pacemakers, the Micra TPS does not require leads or a surgical "pocket" under the skin, so potential sources of complications related to such leads and pocket are eliminated-as are any visible signs of the device.
The Micra design incorporates a retrieval feature to enable retrieval when possible; however, the device is designed to be left in the body. For patients who need more than one device, the miniaturized Micra TPS was designed with a unique feature that enables it to be permanently turned off so it can remain in the body and a new device can be implanted without risk of electrical interaction.
Medtronic said Micra TPS is the first and only leadless pacing system to be approved for both 1.5 and 3 Tesla (T) full-body magnetic resonance imaging (MRI) scans, providing patients with access to the most advanced imaging diagnostic procedures available.
In November 2015, data from the Medtronic Micra TPS Global Clinical Trial were published in the New England Journal of Medicine and presented during a late-breaking Special Report at the American Heart Association Scientific Sessions. Data showed the Micra TPS was successfully implanted in 99.2 percent of patients, there were no (0) dislodgements, and the system met its safety and effectiveness endpoints with wide margins.
The Micra TPS was awarded CE Mark in April 2015 and FDA approval in April 2016. It is intended for use in patients who need a single-chamber pacemaker. The device was designed to allow patients to be followed by their physicians and send data remotely via the Medtronic CareLink Network; remote monitoring of Micra devices is expected to be available later this year.
For more information: www.medtronic.com


 May 31, 2024
May 31, 2024