Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign developed in partnership with Johnson & Johnson for clinicians who manage ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign ...
Jan. 28, 2026 — Corify Care has announced a major development in cardiac electrophysiology with the publication of its ...
Jan. 27. 2026 — Circle Cardiovascular Imaging Inc. has announced the release of cvi42 v6.4, the latest version of its ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 20, 2026 — Kardium Inc. has announced the publication of the PULSAR clinical trial results in the Journal of the ...
Jan. 12, 202 — Abbott and AtaCor Medical have announced a collaboration to advance a next-generation investigational ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
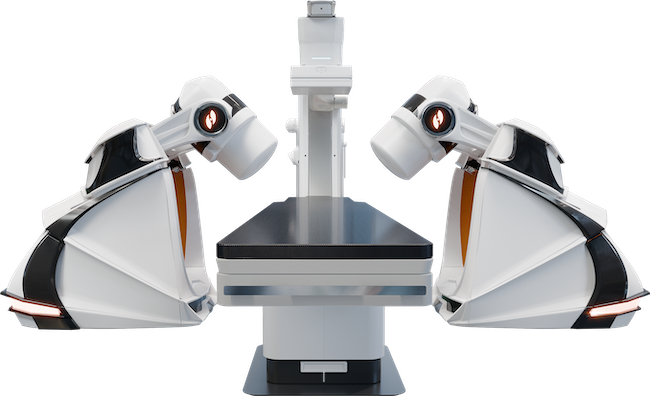
Robotic Magnetic Navigation (RMN) emerged two decades ago as an alternative approach to performing complex ablation ...
Jan. 6, 2026 — Stereotaxis, a supplier of surgical robotics for minimally invasive endovascular intervention, has ...
Dec. 22, 2025 — Abbott recently announced the U.S. Food and Drug Administration (FDA) has approved the company's Volt ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
Dec. 19, 2025 — Johnson & Johnson MedTech has announced its sponsorship of a new data collection platform developed by ...
Dec. 3, 2025 — Recross Cardio Inc., a structural heart company developing next-generation membrane sealing technology ...
Nov. 10, 2025 — Stereotaxis has received U.S. Food and Drug Administration 510(k) clearance for its latest generation ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
Oct. 22, 2025 -- Medical robotics innovator Noah Medical presented two new data sets highlighting the clinical and ...
Oct. 10, 2025 — Johnson & Johnson MedTech, in collaboration with the Heart Rhythm Clinical and Research Solutions, LLC ...
Oct. 10, 2025 — Berlin Heals Holding AG, a clinical-stage medical device company focused on revolutionizing the care of ...






 February 03, 2026
February 03, 2026