Feb. 6, 2026 — Abbott has announced new clinical data from two late-breaking presentations at AF Symposium in Boston (Feb. 5-7, 2026) that demonstrate the strong safety and efficacy of the company's ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
Feb. 6, 2026 — Abbott has announced new clinical data from two late-breaking presentations at AF Symposium in Boston ...
Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Jan. 20, 2026 — Kardium Inc. has announced the publication of the PULSAR clinical trial results in the Journal of the ...
Dec. 19, 2025 — Johnson & Johnson MedTech has announced its sponsorship of a new data collection platform developed by ...
Atrial fibrillation (AFib) is one of the most common heart rhythm disorders, but researchers still don’t have a reliable ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
Sept. 8, 2025 — Pulse Biosciences, Inc., a company leveraging its novel and proprietary Nanosecond Pulsed Field Ablation ...
Sept. 8, 2025 — AMO Pharma Ltd., a clinical-stage specialty biopharmaceutical company focusing on rare genetic ...
Patients with type 2 diabetes (T2D) and chronic kidney disease (CKD) are at elevated risk for cardiovascular disease.1 Y ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
July 7, 2025 — Catheter ablation is a minimally invasive treatment for abnormal heart rhythms. It is often successful in ...
June 13, 2025 — An international study has shown that targeted online education on atrial fibrillation (AF) for health ...
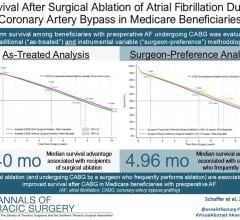
June 4, 2025 — A new study published in The Annals of Thoracic Surgery, a journal from The Society of Thoracic Surgeons ...
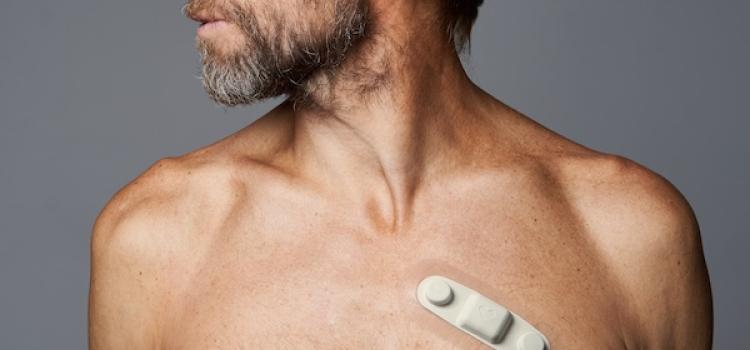
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 21, 2025 — Corify Care recently announced it has entered into a know-how agreement with Mayo Clinic. Corify Care is ...
April 25, 2025 – Medtronic plc, a global leader in healthcare technology, recently received U.S. Food and Drug ...
April 27, 2025 – The Heart Rhythm Society (HRS) has announced the findings of new studies demonstrating the safety and ...





 February 06, 2026
February 06, 2026