July 24, 2024 — During the American Heart Association Basic Cardiovascular Sciences Scientific Sessions 2024, BCVS ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
July 24, 2024 — Volta Medical, a health technology company developing artificial intelligence (AI) solutions to assist ...
July 17, 2024 — BioCardia, Inc., a company focused on cellular and cell-derived therapeutics for the treatment of ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
July 16, 2024 — Ultrahuman, a pioneer in wearable technology, launches PowerPlugs, a platform for individual apps and ...

In a room of 20 people, it’s likely that about 10 of them, or half, will presently have some form of cardiovascular ...
July 8, 2024 — Pulsed field ablation (PFA) is safe for treating patients with common types of atrial fibrillation (AF) ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
June 19, 2024 — When electrophysiologist Eugenio Cingolani, MD, isn’t seeing patients, he can usually be found in his ...

While the spotlight in 2023 shone on developments around Generative Artificial Intelligence (AI), away from the ...
June 12, 2024 — A team of Ochsner Health cardiologists recently published an article in the Journal of the American ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...

Innovation in medical technology exists to improve patient outcomes, increase surgical efficiency and enhance patient ...

June 7, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of the ...

Stroke recovery is a challenging process that extends for months after hospital discharge. Issues like cognitive ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 24, 2024 — An artificial intelligence (AI) program developed by investigators in the Smidt Heart Institute and their ...
May 18, 2024 — The Heart Rhythm Society (HRS) has announced the findings of three new studies demonstrating the safety ...
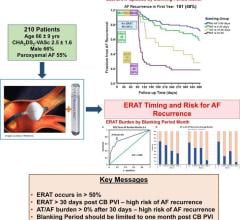
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period ...


 July 05, 2024
July 05, 2024