Nov. 12, 2025 — Siemens has announced plans to deconsolidate its remaining stake in Siemens Healthineers (currently circa 67 percent). The company plans to transfer 30 percent of Siemens Healthineers ...
Cardiovascular Business
The cardiovascular business channel offers news and new technologies to aid the business management of the cardiovascular service line.
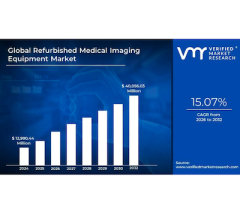
Jan. 11, 2026 — The Global Refurbished Medical Imaging Equipment Market Size is projected to grow at a CAGR of 15.07% ...
Nov. 12, 2025 — Siemens has announced plans to deconsolidate its remaining stake in Siemens Healthineers (currently ...
Oct. 23, 2025 — At TCT 2025, the Cardiovascular Research Foundation (CRF) announced the 2025 SET-10 (Scientific ...
Many cardiology departments face significant challenges meeting regulatory requirements, quality metrics, and ...
Oct. 27, 2025 — The American Heart Association (Association) has launched its latest professional certification program ...
Aug. 26, 2025 — Octane, an innovative organization building the SoCal of Tomorrow by connecting people, resources and ...
The American Heart Association recently announced that Dr. Christine E. Seidman, an internationally renowned physician ...
Jeff Rieder M.D., a cardiologist with Coastal Cardiology Charleston, clearly recalls the time when reporting was ...

Cardiology practices face mounting financial pressures — rising operational costs, declining reimbursements, and complex ...
As part of DAIC's continuing Thought Leadership Series, this month Editorial Director Melinda Taschetta-Millane sits ...
In DAIC's continuing Thought Leadership Series with Dr. Jeffrey Soble, a practicing cardiologist at Rush University ...
The ability of a cardiologist to work remotely can save lives. By leveraging the advantages of a cloud-based ...
July 29, 2024 — Edwards Lifesciences announced investments that reflect the company’s deep commitment to advancing ...
In this segment of our DAIC Thought Leadership Series, which features one-on-one conversations with cardiovascular ...
July 26, 2024 — VentriJect, a Danish medtech start-up seeking to revolutionize the way cardiorespiratory fitness (CRF) ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
Industry trade shows and conferences seem to be making their comeback in 2024. And the Healthcare Information and ...
July 23, 2024 — The Society of Cardiovascular Computed Tomography (SCCT) announced its best original science award ...
July 19, 2024 — The Society of Cardiovascular Computed Tomography (SCCT) received near-record breaking submissions of ...





 January 23, 2026
January 23, 2026