Feb. 25, 2026 — Gentuity LLC, a medical technology company specializing in next-generation intravascular imaging devices, has announced a commercial collaboration with GE HealthCare to further improve ...
March 4, 2026 — HeartBeam, Inc. has announced a commercial partnership with ClearCardio. In addition to serving as ...
March 2, 2026 — Ascend Cardiovascular has launched its re-imagined pediatric cardiology product, a comprehensive ...
March 4, 2026 — Inflo Health has launched a comprehensive cardiology suite designed to address care management gaps ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
March 4, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has announced Late-Breaking clinical ...
March 3, 2026 — BioCardia, Inc., a developer of cellular and cell-derived therapeutics for treating cardiovascular and ...
March 3, 2026 — MedDream will present its cloud-native, AI-ready universal DICOM viewer in the Amazon Web Services (AWS) ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Feb. 27, 2026 — The U.S. Food and Drug Administration (FDA) has approved Abbott’s CardioMEMS Hero device — a pulmonary ...
Feb. 25, 2026 — Gentuity LLC, a medical technology company specializing in next-generation intravascular imaging devices ...
Feb. 26, 2026 — Eko Health has partnered with Wayne General Hospital in Wayne County, Mississippi, to deploy SENSORA, an ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
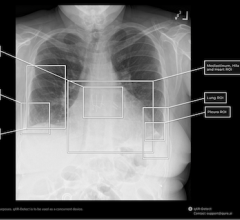
Feb. 26, 2026 — The U.S. Food and Drug Administration (FDA) has given 510(k) class II clearance of qXR-Detect, the ...
Feb. 10, 2026 – AccurKardia, a provider of ECG-based diagnostics technology, recently announced results from a new study ...
Feb. 19, 2026 — Preliminary results from an Italian registry describe the risk profile of women experiencing acute ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Feb. 18, 2026 — Researchers at the Mount Sinai Kravis Children’s Heart Center led a multicenter effort to develop and ...
The American Society of Radiologic Technologists (ASRT) will host a free Virtual Career Fair on March 17, from 4-7 p.m ...
Feb. 14, 2026 — Cardiovascular Research Foundation (CRF) has announced the creation of Complex Coronary Summit, a new ...




 March 06, 2026
March 06, 2026