Jan. 12, 2026 — YorLabs, Inc., a medical technology company providing next-generation intracardiac imaging solutions for ...
Jan. 12, 202 — Abbott and AtaCor Medical have announced a collaboration to advance a next-generation investigational ...
Jan. 12, 2026 — HeartLung Corp. has announced U.S. Food and Drug Administration (FDA) clearance of AI-CVD, its AI ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
Jan. 6, 2026 — Millions of Americans living with heart failure are not receiving medications that have been proven for ...
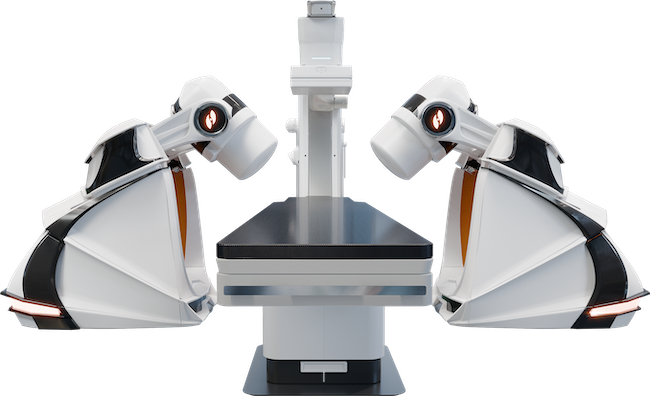
Robotic Magnetic Navigation (RMN) emerged two decades ago as an alternative approach to performing complex ablation ...
Jan. 9, 2026 — Effective January 1, 2026, Elucid's Plaque-IQ coronary plaque analysis has received a new Category I ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Jan. 7, 2026 — UltraSight, a provider of AI-guided cardiac imaging workflows, has partnered with Jefferson Health, one ...
Jan. 6, 2026 — Cleerly, a leader in AI-based cardiovascular imaging, has announced that Aetna will begin covering ...
Dec. 10, 2025 — Medtronic plc has announced the first commercial use of the Liberant thrombectomy system (Liberant) ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Jan. 6, 2026 — W. L. Gore & Associates’ medical business (Gore) has announced the FDA approval of the Gore Viabahn ...
Jan. 6, 2026 — Stereotaxis, a supplier of surgical robotics for minimally invasive endovascular intervention, has ...
Jan. 5, 2026 — Medera Inc., a clinical-stage biopharmaceutical company focused on targeting cardiovascular diseases by ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Dec. 22, 2025 — Abbott recently announced the U.S. Food and Drug Administration (FDA) has approved the company's Volt ...
Dec. 12, 2025 — Increased volume of epicardial adipose tissue, detected by cardiovascular imaging, was found to be ...
Dec. 26, 2025 — Edwards Lifesciences has announced the company’s SAPIEN M3 mitral valve replacement system, a ...


 January 13, 2026
January 13, 2026

















