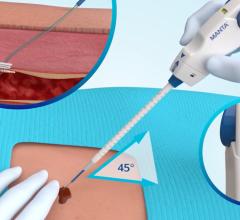
June 12, 2025 — Viz.ai recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for Viz Subdural Plus, the first and only comprehensive solution for quantifying the ...
Hemostasis Management
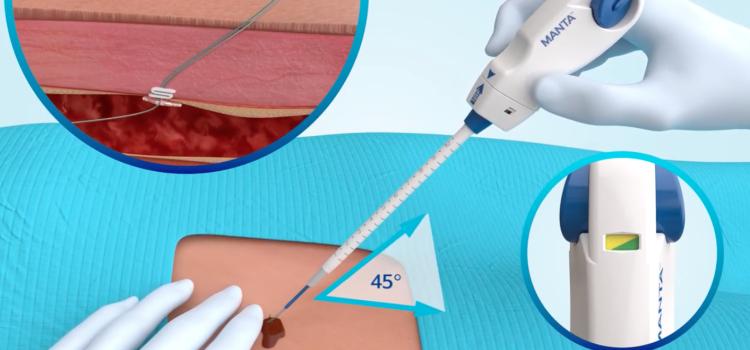
This channel includes news and new technology innovations for devices to help stop bleeding at vascular access sites. These include coated or treated bandages to accelorate or augment the clotting cascade, vascular closure devices and compression devices, including radial artery compression systems.
June 12, 2025 — Viz.ai recently announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
October 27, 2021 — Teleflex Inc. announced the completion of patient enrollment in a clinical study evaluating the ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
Arnold Seto, M.D., MPA, FSCAI, chief of cardiology, Long Beach Veterans Affairs Medical Center and director ...
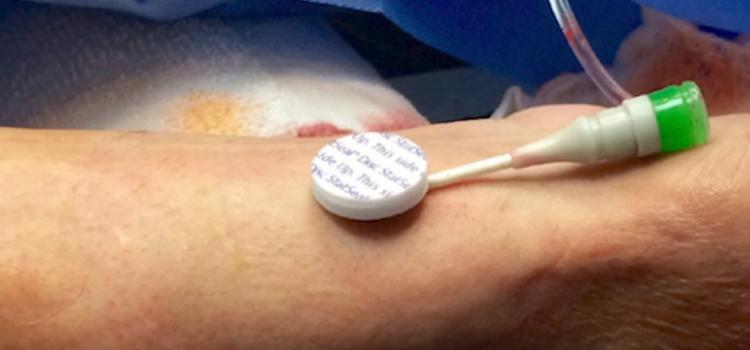
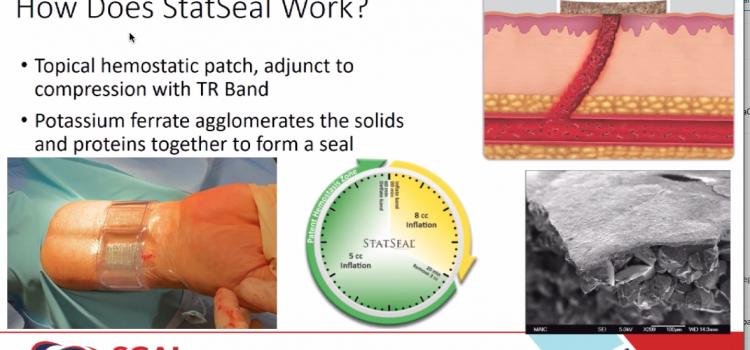
April 28, 2021 — A new study reveals the use of a potassium ferrate hemostatic patch (PFHP) reduces the time to ...
April 21, 2021 — EnsiteVascular announced it received its second U.S. Food and Drug Administration (FDA) market ...

While many cardiac and vascular procedures have largely moved to minimally invasive techniques, the size of these ...
February 8, 2021 — Teleflex Inc. said it recently completed its acquisition of Z-Medica LLC, an industry-leading ...
August 25, 2020 – Atlantic Health System’s Morristown Medical Center has opened one of the region’s first radial lounges ...

Vascular access site bleeding is associated with higher complications and mortality rates. For decades femoral access ...
June 8, 2020 – BD (Becton, Dickinson and Company) launched the Halo One Thin-Walled Guiding Sheath, designed to perform ...
December 30, 2019 — Merit Medical Systems Inc., a leading manufacturer of proprietary devices used primarily in ...
Ashish Pershad, M.D., chief of interventional cardiology, Banner University Medical Center, Phoenix, explains the trend ...
August 29, 2019 – Merit Medical Systems Inc. announced the U.S. commercial launch of the PreludeSync Evo radial ...

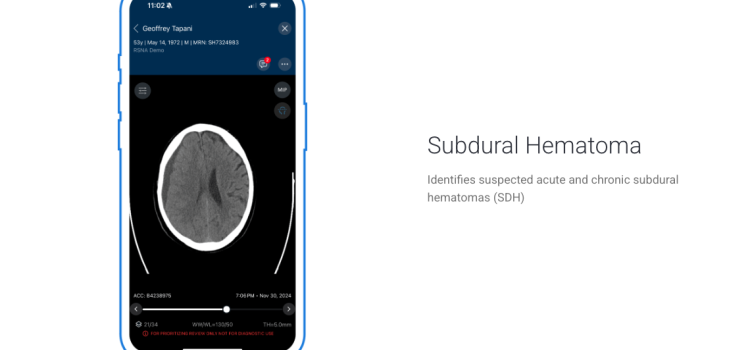
 June 12, 2025
June 12, 2025