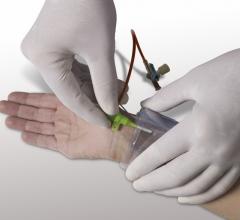
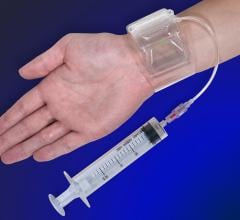
October 7, 2013 — Merit Medical Systems Inc. announced that it has acquired Datascope Corp.’s Safeguard Pressure ...
Hemostasis Management
This channel includes news and new technology innovations for devices to help stop bleeding at vascular access sites. These include coated or treated bandages to accelorate or augment the clotting cascade, vascular closure devices and compression devices, including radial artery compression systems.
There is no shortage of debate among interventional cardiologists over whether vascular closure devices should be used ...
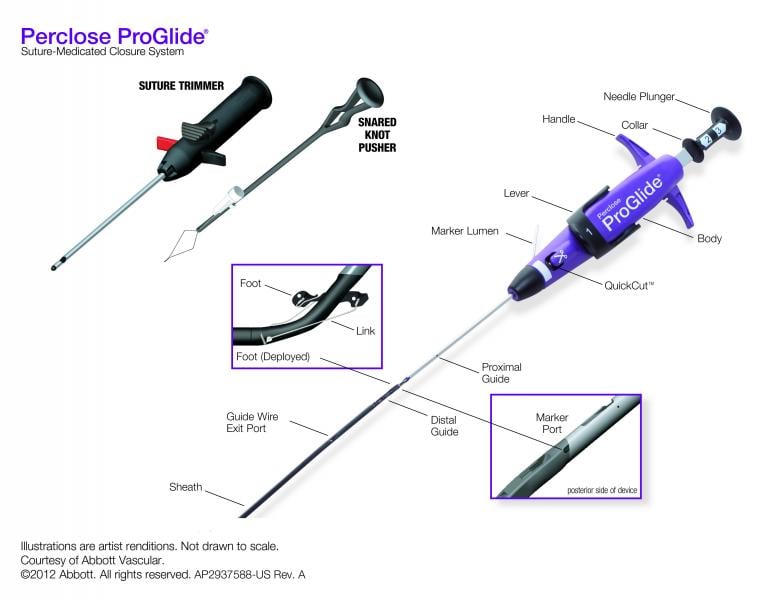
Vascular closure devices (VCD) were first introduced in the early 1990s with a goal to achieve quick hemostasis, thus ...
August 8, 2013 — The U.S. Food and Drug Administration (FDA) accepted Biomedica’s Investigational New Drug (IND) ...
May 28, 2013 — The U.S. District Court for the District of New Jersey approved an agreement under which Vascular ...
April 16, 2013 — Maquet Cardiovascular LLC has received 510(k) clearance from the U.S. Food and Drug Administration (FDA ...
March 27, 2013 — The U.S. Food and Drug Administration (FDA) notified healthcare professionals of a Class I recall of ...
March 6, 2013 — Terumo Medical Corp. announced it filed a patent infringement lawsuit against Vascular Solutions Inc ...
February 7, 2013 — Cardiva Medical Inc. announced that the U.S. Food and Drug Administration (FDA) has granted Premarket ...
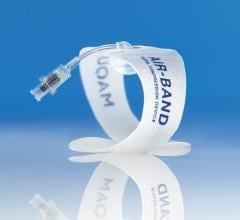
November 6, 2012 — Vascular Solutions launched the R-Band radial hemostasis compression device in the United States ...
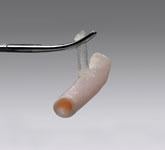
Vascular closure devices that use an active method to immediately seal the femoral access site can enable faster patient ...
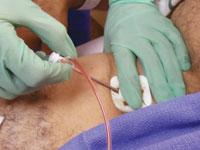
The implementation of dedicated access site surveillance and educational programs, in tandem with pre-existent ...
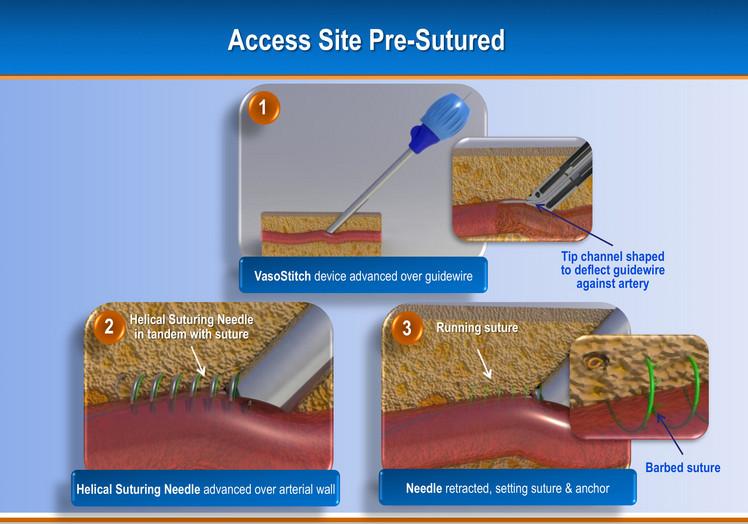
Due to the increasing number of transcatheter aortic valve replacements (TAVR) and endovascular aneurysm repair (EVAR) ...
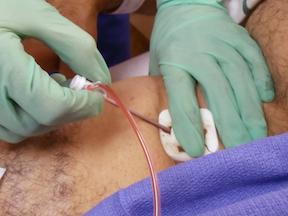
Percutaneous coronary interventional (PCI) procedures are performed throughout hundreds of US institutions every day ...
July 6, 2012 — Vascular Closure Systems Inc. announced that the 30-day follow-up results for the Phase I first-in-human ...

 October 07, 2013
October 07, 2013