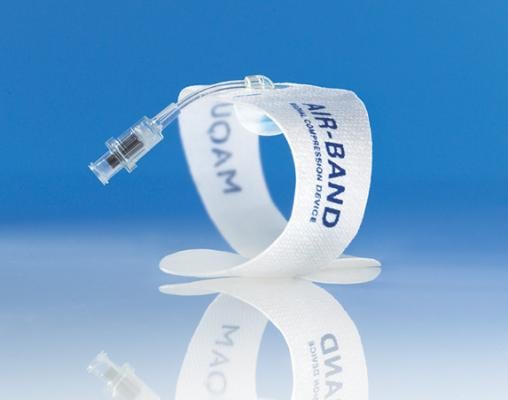
April 16, 2013 — Maquet Cardiovascular LLC has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and European CE mark approval for its new Air-Band Radial Compression Device. Indicated to assist hemostasis of the radial artery after a transradial procedure, the device is designed to compress the radial artery puncture site while maintaining site visibility and a secure fit around the wrist. Air-Band will be commercially available in the European Union later this month and in the United States in April.
"We have found that Air-Band is easy to use, reliable and effective in achieving hemostasis following radially accessed coronary catheterization procedures," said Christian Valina, M.D., interventional cardiologist at the University Heart Centre of Bad Krozingen in Germany. "My colleagues and I were impressed by the secure fit around the wrist that the adhesive band provides. We believe that Air-Band represents important progress towards ensuring patient safety and comfort during these important procedures."
"Drawing on our significant experience in hemostasis management, we designed Air-Band to provide all the valued benefits of our Safeguard Pressure Assisted Device — which assists in obtaining and maintaining hemostasis after a femoral procedure — and applied our knowledge to a radial application," said Christian Keller, president and CEO of Maquet Cardiovascular. "Both devices simplify hemostasis management by delivering hands-free adjustable pressure, offering simple application and removal, and providing clear site visibility and assessment without compromising patient comfort, which is a priority for us."
Air-Band is a 26-cm long, latex-free, self-adhesive wristband with a clear window and bulb that facilitate visualization of the puncture site. A luer valve on the end of the clear fill tube enables any standard syringe to be connected to inflate and deflate the bulb with air to provide compression of the radial puncture site.
With its textile wristband, Air-Band contributes to patient comfort, as it neither cuts into the skin nor has protruding plastic parts. The adhesive enables a secure fit around all patient wrists, avoiding movement and dislocation. Both the size and shape of Air-Band’s inflatable bulb minimize the compression risk to surrounding nerve structures or areas other than the radial puncture site. The standard luer valve allows easy inflation and deflation with any standard luer syringe.
For more information: www.maquet.com


 November 14, 2025
November 14, 2025 









