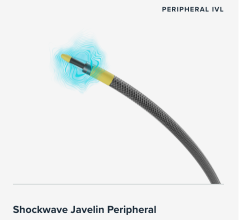
Since receiving FDA approval in 2016, intravascular lithotripsy (IVL) systems have grown in popularity among interventionalists for treating calcified plaque inside coronary and peripheral arteries ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic ...
Dec. 10, 2025 — Medtronic plc has announced the first commercial use of the Liberant thrombectomy system (Liberant) ...
Dec. 15, 2025 — Royal Philips has entered into an agreement to acquire SpectraWAVE, Inc., an innovator in enhanced ...
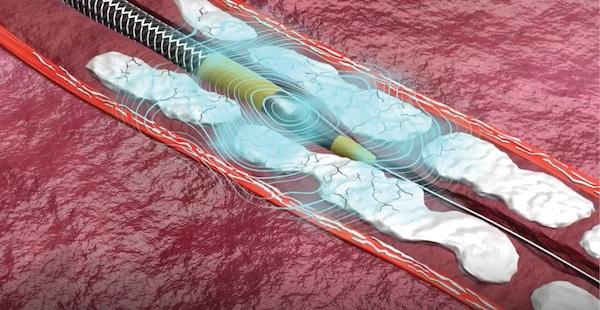
Since receiving FDA approval in 2016, intravascular lithotripsy (IVL) systems have grown in popularity among ...
Nov. 4, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on treating calcified arterial disease ...
Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave ...
Sept. 9, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on safely and effectively treating ...
June 6, 2025 – A large retrospective review of more than 100,000 patients found the use of glucagon-like peptide-1 ...
Feb. 3, 2025 — Today, Peripheral Artery Disease (PAD) affects 10 million Americans and is the most common cause of limb ...
Jan. 13, 2025 – R3 Vascular Inc., a medical device company dedicated to treating peripheral arterial disease (PAD) ...
A new study reports persistent disparities in outcomes for people with peripheral artery disease (PAD) and its more ...
Dec. 13, 2024 – Sensome has announced positive results from two studies of its Clotild Smart Guidewire System ...
Nov. 18, 2024 — In November, Esperion presented an analysis from the CLEAR Outcomes study focused on patients with ...
Nov. 4, 2024 — Royal Philips recently announced enrollment of the first patient in the U.S. THOR IDE clinical trial ...
Sept. 16, 2024 – Shockwave Medical, Inc., part of Johnson & Johnson MedTech, has announced the full U.S. launch of the ...

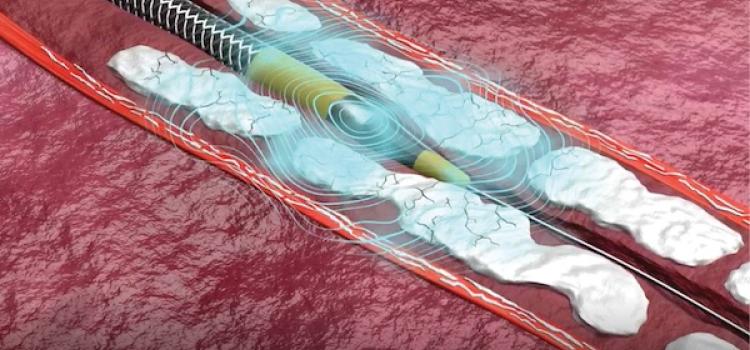

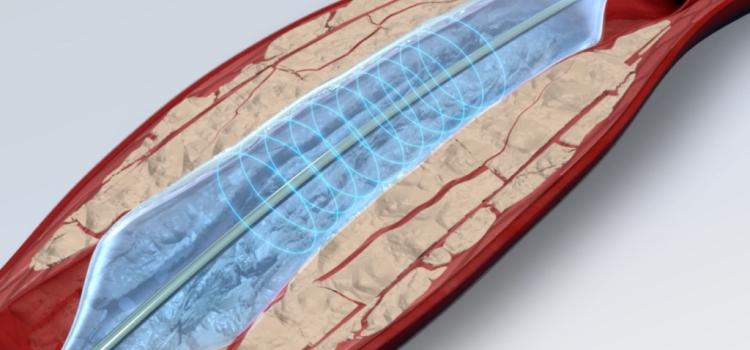

 January 27, 2026
January 27, 2026