Feb. 3, 2026 — The first official summit of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) organized directly under the umbrella of the European Society of Cardiology ...
Cardiovascular Surgery
This channel includes news and new technology innovations for cardiac surgery. This includes coronary artery bypass grafts (CABG) and surgical valve replacements.
Feb. 6, 2026 — Robert Wood Johnson University Hospital (RWJUH), in partnership with Rutgers Robert Wood Johnson Medical ...
Feb. 3, 2026 — The first official summit of the European Association of Percutaneous Cardiovascular Interventions (EAPCI ...
Jan. 31, 2026 — The Society of Thoracic Surgeons (STS) has elected Vinay Badhwar, MD, as its 62nd president during the ...
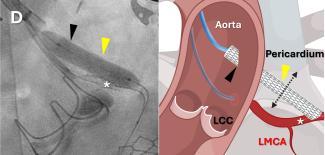
In a world first, a team of researchers at the National Institutes of Health (NIH) and Emory School of Medicine, Atlanta ...
Nov. 11, 2025 -— Integra LifeSciences Holdings Corp. has announced the FDA 510(k) clearance for use of its CUSA Clarity ...
Nov. 8, 2025 — Sanjay Rajagopalan, MD, MBA, Chief of Cardiovascular Medicine at University Hospitals Harrington Heart & ...
Sept. 8, 2025 — Pulse Biosciences, Inc., a company leveraging its novel and proprietary Nanosecond Pulsed Field Ablation ...
Aug. 13, 2025 — A recent study published in the Journal of the Society for Cardiovascular Angiography & Interventions ...
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...
Jan. 29, 2025 – Scandinavian Real Heart AB (recently aannounced that its total artificial heart, Realheart TAH, has been ...
Jan. 24, 2025 — In-patients undergoing coronary artery bypass grafting (CABG), a novel analysis evaluating surgeon ...
Oct. 23, 2024 – The Society for Vascular Surgery (SVS) is launching a three-year patient education campaign, Highway to ...
July 10, 2024 — Reviva Pharmaceuticals Holdings, Inc., a late-stage pharmaceutical company developing therapies that ...
July 3, 2024 — Jon Kobashigawa, MD, director of the Heart Transplant Program in the Department of Cardiology in the ...
June 13, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...



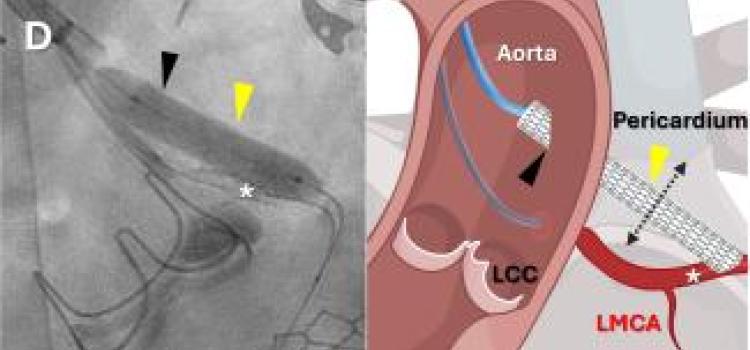


 February 06, 2026
February 06, 2026












