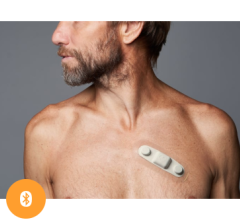
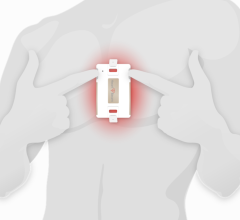
Jan. 13, 2026 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced a strategic collaboration with Biobeat Technologies, Ltd. to expand diagnostic ...
Wearables
This channel includes news and new technology innovations for wearable cardiac diagnostic systems. These include technologies related to smart phone apps, wearable monitors, consumer-grade wearables and medical grade FDA cleared wearables. A good overview on the future of these technologies can be found in this article.
Jan. 13, 2026 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced a ...
Dec. 10, 2025 — The Singapore-MIT Alliance for Research and Technology (SMART), Massachusetts Institute of Technology’s ...
Nov. 10, 2025 — Kestra Medical Technologies, a wearable medical device and digital healthcare company, has announced ...
Technology-enabled “Insourcing” approach to cardiac arrhythmia diagnosis provides patient and provider benefits The ...
Oct. 21, 2025 — Early and accurate detection of heart rhythm problems can mean the difference between life-saving ...
July 30. 2025 — Cardiosense, a medical AI company, recently announced that the U.S. Food and Drug Administration (FDA) ...
July 15, 2025 — Vivalink, a provider of digital healthcare solutions, has announced its wearable ECG monitor is ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
July 16, 2024 — Ultrahuman, a pioneer in wearable technology, launches PowerPlugs, a platform for individual apps and ...
July 15, 2024 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care through the power of ...

While the spotlight in 2023 shone on developments around Generative Artificial Intelligence (AI), away from the ...
COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...
May 17, 2024 — Royal Philips, a global leader in health technology, is presenting new retrospective study results ...
April 22, 2024 — At the annual American College of Cardiology conference (ACC.24) in Atlanta last week, RCE Technologies ...
April 12, 2024 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care through the power ...
COVID-19 has posed challenges for physicians whose cardiac patients are at-risk and reluctant to schedule an office ...
February 28, 2024 — Artificial intelligence (AI) may transform cardiovascular medicine. For now, though, many challenges ...
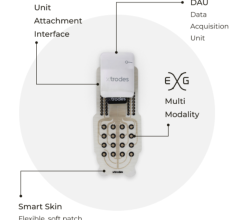
February 20, 2024 — X-trodes, a bio-convergence company bringing wireless monitoring solutions to the home environment ...
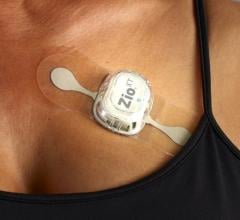
January 3, 2024 — iRhythm Technologies, Inc., a leading digital health care company focused on creating trusted ...





 January 14, 2026
January 14, 2026