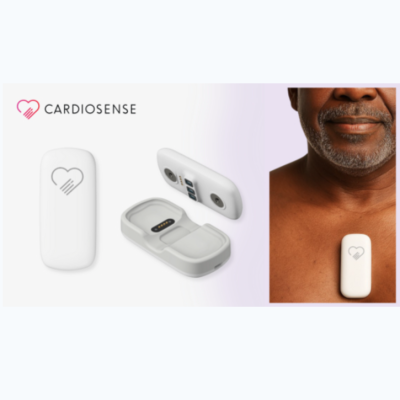
July 30. 2025 — Cardiosense, a medical AI company, recently announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the CardioTag device, a multimodal, wearable sensor that simultaneously captures high-fidelity electrocardiogram (ECG), photoplethysmogram (PPG), and seismocardiogram (SCG) signals, empowering clinicians and patients with a comprehensive noninvasive solution to assess cardiac function across care settings.
“Our team is thrilled to achieve this critical milestone as part of our mission to advance cardiac care,” said Amit Gupta, Co-founder and CEO of Cardiosense. “Traditionally, noninvasive cardiac monitoring has primarily focused on ECG and rhythm analysis. With the CardioTag device, we are adding an entirely new dimension by also capturing physiological data on cardiac mechanics and blood flow, providing unprecedented visibility into a patient’s cardiac function, hemodynamics, and volume status.”
The FDA clearance authorizes the CardioTag device for noninvasive measurement of SCG, ECG, and PPG signals as well as heart rate (HR) and pulse rate (PR). SCG is a noninvasive technique that measures subtle vibrations on the chest wall associated with cardiac mechanical activity. Clinical studies conducted by Cardiosense have demonstrated that analyzing the SCG waveform alongside ECG and PPG signals can be used to accurately assess cardiac timing intervals such as left ventricular ejection time (LVET)—a measure of how efficiently the heart is pumping blood—compared to the current standard-of-care. Cardiosense will begin exploring pilots with the CardioTag device paired with AI algorithms using the SCG, ECG, or PPG data from the device.
“The CardioTag clearance marks a pivotal step toward clinical adoption and broader access to pressure-guided treatment,” said Andrew Carek, Co-founder and CTO of Cardiosense. “We’re excited for the foundational role that the CardioTag device will play in building a noninvasive cardiac AI platform, as the signals it collects provide a rich data input upon which AI models for cardiovascular parameters can be developed, such as our pulmonary capillary wedge pressure (PCWP) algorithm.”
A recent prospective, multicenter study, published in the Journal of the American College of Cardiology: Heart Failure and presented as Late-Breaking Science at the American Heart Association’s 2024 Scientific Sessions, demonstrated that Cardiosense’s AI algorithm for PCWP, which received FDA Breakthrough Device designation, could estimate PCWP values with accuracy on par with implantable hemodynamic sensors in patients with heart failure with reduced ejection fraction (HFrEF). Upon regulatory approval for the PCWP Analysis Software, the algorithm will be paired with the CardioTag device for advanced heart failure management.
The CardioTag device was built on years of clinical research and cross-disciplinary innovation spanning biomedical engineering, cardiovascular medicine, and data science. “This is a deeply meaningful milestone. For over a decade, we’ve worked to turn a bold idea into a clinically reliable, noninvasive technology that truly meets patients where they are. With FDA clearance, we’re taking a major step toward bringing precision hemodynamic insights into everyday patient care—no matter the setting,” said Omer Inan, PhD, Co-founder and Chief Scientific Officer at Cardiosense.
For more information visit www.cardiosense.com.


 January 15, 2026
January 15, 2026 









