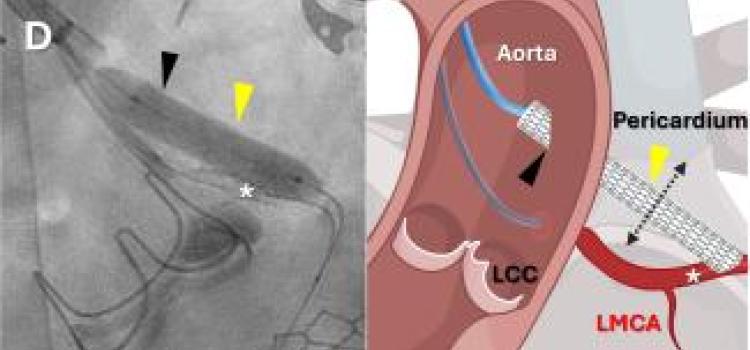
In a world first, a team of researchers at the National Institutes of Health (NIH) and Emory School of Medicine, Atlanta, has successfully performed a coronary artery bypass — a normally open-heart ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
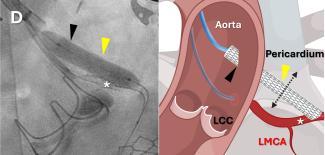
In a world first, a team of researchers at the National Institutes of Health (NIH) and Emory School of Medicine, Atlanta ...
Dec. 26, 2025 — Edwards Lifesciences has announced the company’s SAPIEN M3 mitral valve replacement system, a ...
Dec. 18, 2025 — Abbott has received U.S. Food and Drug Administration (FDA) clearance and CE Mark for its Amplatzer ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Nov. 17, 2025 — Royal Philips has introduced DeviceGuide, an AI-powered device tracking* solution that assists ...
Oct. 28, 2025 — People who underwent a minimally invasive procedure to have their heart’s aortic valve replaced had ...
Oct. 25, 2025 — Medtronic plc has announced the launch of the Stedi Extra Support guidewire, designed to enhance ...
Sept. 23, 2025 — CeleCor Therapeutics’ multinational Phase 3 clinical trial of its investigational heart-attack drug ...
Sept. 22, 2025 — The latest findings on heart failure (HF) published by the Heart Failure Society of America (HFSA) reve ...
Aug. 28, 2025 — Medtronic plc has announced it received U.S. Food and Drug Administration (FDA) approval for the ...
Aug. 18, 2025 — A new study published in The Annals of Thoracic Surgery, a journal from The Society of Thoracic Surgeons ...
Aug. 4, 2025 — Corcym announced that its Perceval Plus sutureless aortic heart valve was used in a first-ever robotic ...
July 10, 2025 — On July 2, 2025, Centers for Medicare & Medicaid Services (CMS) issued a National Coverage Determination ...
June 27, 2025 – Foldax Inc., a leader in the development of innovative polymer heart valves, has announced compelling ...
June 04, 2025 — HeartSciences Inc. has announced that the U.S. Food and Drug Administration has granted Breakthrough ...
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...


 January 15, 2026
January 15, 2026














