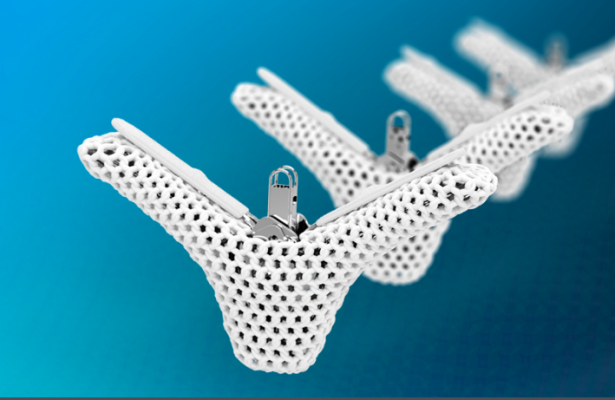
July 10, 2025 — On July 2, 2025, Centers for Medicare & Medicaid Services (CMS) issued a National Coverage Determination (NCD) that covers Abbott's FDA approved Transcatheter Edge-to-Edge Repair for Tricuspid Valve Regurgitation (T-TEER) for the treatment of symptomatic tricuspid regurgitation (TR) under Coverage with Evidence Development (CED), when furnished in accordance with the coverage criteria specified in the NCD. The complete NCD decision memorandum is available on our website.
Below is a list of studies that have been determined to meet the requirements for coverage under CED.
Study Title: TRIClip CoverAge with Evidence Development (CED) Real-World Evidence (RWE) Study (TRICARE)
Sponsor: Abbott
Clinicaltrials.gov number: NCT06920745
CMS Approval Date: 07/08/2025
Abbott’s TriClip G5 device, the only FDA-approved T-TEER device, will now be covered by Medicare, ensuring long-term, predictable access to a minimally invasive treatment option for eligible Medicare and Medicare Advantage beneficiaries. This important decision marks a pivotal development in expanding access to a potentially lifesaving treatment for the approximately 1.6 million people in the U.S. with moderate or severe TR who currently have limited options.
For many patients who are not candidates for open-heart surgery, TriClip offers a path to significantly improved quality of life and a reduction in heart failure hospitalizations.
The final NCD coincides with the U.S. launch of Abbott's TriClip G5 and MitraClip G5 systems that incorporate advanced features designed for easy handling, precise positioning, and intuitive deployment -— further enhancing their effectiveness in mitral and tricuspid valve repair procedures. The MitraClip G5 and TriClip G5 systems recently received FDA approval and CE Mark (in Europe).


 January 15, 2026
January 15, 2026 









