Nov. 4, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on treating calcified arterial disease, presented the results of the first 95 patients treated in the POWER PAD II U.S ...
VIVA
This channel contains news about the annual Vascular Interventional Advances (VIVA) conference. VIVA is a premier conference for new techniques and technologies to treat vascular disease. This includes the areas of peripheral artery disease (PAD), critical limb ischemia (CLI), endovascular repair and venous therapies.
Nov. 4, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on treating calcified arterial disease ...
Nov. 3, 2025 — Penumbra, Inc. has announced additional results of the STORM-PE randomized controlled trial (RCT), which ...
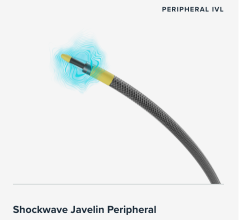
Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave ...
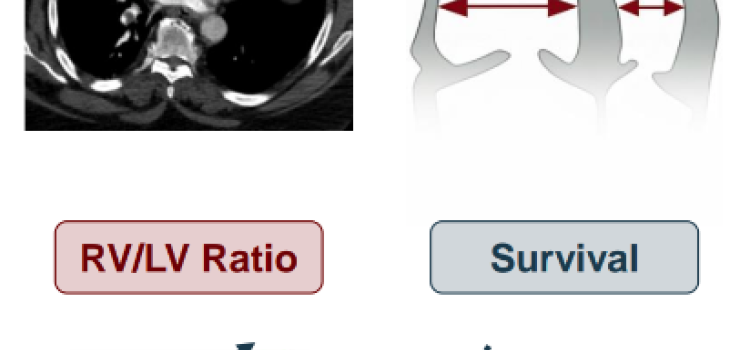
Oct. 27, 2025 – Penumbra, Inc. has announced the results of the STORM-PE randomized controlled trial (RCT), which found ...
Sept. 9, 2025 — Amplitude Vascular Systems (AVS), a medical device company focused on safely and effectively treating ...
November 9, 2023 — The VIVA Foundation, a not-for-profit organization dedicated to advancing the field of vascular ...
November 2, 2023 — The latest STRIKE-PE data, presented by Penumbra, Inc. at the Vascular Interventional Advances ...
November 2, 2023 — Contego Medical Inc. announced the presentation of late-breaking clinical results from the ...
November 2, 2023 — The second round of late-breaking clinical trials results from the Vascular InterVentional Advances ...
November 1, 2023 — The VIVA Foundation has announced the results for the first Late-Breaking Clinical Trial sessions at ...
October 31, 2023 — Results of four late-breaking clinical trials presented during the The VEINS Conference 2023, taking ...
November 3, 2022 — Medtronic, a global leader in medical technology, today announced the results of two clinical studies ...
November 2, 2022 — The second round of late-breaking clinical trial results were announced at VIVA22 on Nov. 1 in Las ...
November 2, 2022 — A number of awards of distinction were presented during The VEINS (Venous Endovascular INterventional ...
October 31, 2022 — Results from the Late-Breaking Clinical Trials session at The VEINS Conference in Las Vegas, NV, were ...




 November 09, 2025
November 09, 2025