Photo: Shockwave Medical
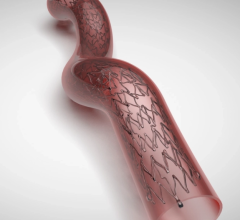
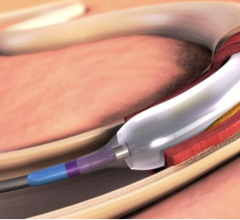
Nov. 4, 2025 – Johnson & Johnson MedTech has announced the one-year results in patients treated with its Shockwave Javelin Peripheral IVL Catheter, a novel Forward IVL platform designed to modify calcified occlusive or extremely narrowed lesions in patients with peripheral artery disease (PAD). The results, presented as a late-breaking clinical trial at the annual Vascular InterVentional Advances (VIVA) meeting, demonstrate low rates of major amputation and cardiovascular death in a high-risk, complex patient population.
“These one year outcomes show that Shockwave Javelin demonstrated lasting durability, with most patients remaining free from repeat intervention,” said JD Corl, M.D., F.A.C.C., F.S.C.A.I., Medical Director of the PAD/CLI Program at The Lindner Center for Research and Education at The Christ Hospital and Principal Investigator of the FORWARD PAD study.i “Severe calcification has long been one of the greatest challenges in endovascular treatment of PAD, driving higher rates of complications, mortality and limb loss. Until now, clinicians lacked a technology that could modify calcium safely to enable the crossing of heavily stenosed lesions. These results demonstrate that IVL is not just overcoming that barrier—it is redefining what’s possible and enabling optimized outcomes for a broader population of PAD patients.”
Key findings from the one-year data analysis include:
- Low Rates of Major Amputation: The 12-month rate of target limb major amputation was 1.0%.
- Low Rates of Cardiovascular Death: At one year, the cardiovascular death rate was 3.9%.
- CD-TLR Rate of 14.7%.
- Durable Patency: At one-year, primary patency above-the-knee (ATK) was 72.7% and below-the-knee (BTK) 61.5%.
“These one-year data strengthen our conviction in Javelin as a safe, effective solution for modifying and crossing the most complex PAD lesions,” said Nick West, M.D., Chief Medical Officer at Shockwave Medical. “The durable benefits we’re seeing — specifically in difficult-to-cross, severely calcified disease—signal a step change in how clinicians can approach these cases. We remain committed to advancing innovations that expand options and elevate outcomes for PAD patients.”
Peripheral artery disease is the narrowing or blockage of the vessels that carry blood from the heart to the legs, reducing blood flow and affecting more than 12 million people in the U.S. alone.1 People suffering from PAD have an impaired quality of life and increased risk of heart attack or stroke.2 Chronic limb-threatening ischemia is the most advanced and serious form of PAD, impacting nearly 2 million patients in the U.S. It is associated with 40% major amputations at one year and a 50% mortality rate at five years,3 worse than many forms of cancer.4
The feasibility and IDE studies of the Shockwave Javelin IVL catheter, MINI S and FORWARD PAD, respectively, were prospective, multi-center, single-arm, angiographic core-lab adjudicated studies with similar inclusion and exclusion criteria. The studies enrolled 110 patients, with 103 with heavily calcified, stenotic peripheral arterial lesions. The average lesion length was 77mm, just under half of the target lesions were located below the knee, and over a third were chronic total occlusions.
Learn more at www.shockwavemedical.com and thenext.jnjmedtech.com.
iDr. Corl is a paid consultant for Shockwave Medical. He has not been compensated in connection with this press release.


 November 09, 2025
November 09, 2025