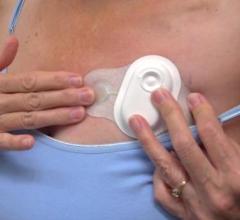
Sept. 25, 2025 — Royal Philips has entered a national partnership in the United States with Optum Healthcare. The inclusion of Philips’ Mobile Cardiac Telemetry (MCOT) and Philips Extended Holter ...
Remote Monitoring
Sept. 25, 2025 — Royal Philips has entered a national partnership in the United States with Optum Healthcare. The ...
Sept. 16, 2025 — PaceMate has appointed Sean Shoffstall as Head of AI, Innovation and Data Strategy. Shoffstall joins ...
August 14, 2025 — PaceMate has officially completed a year-long project, resulting in two new exclusive abilities. The ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

As healthcare systems continue to shift toward value-based care and proactive disease management, remote monitoring has ...
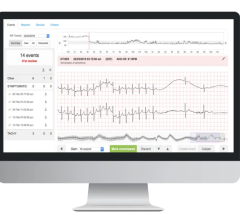
April 28, 2025 — iRhythm Technologies, Inc. recently announced results from a large real-world retrospective analysis ...

Imagine a future where a silent, undetected heart condition is caught before it turns into a life-threatening event — a ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
July 31, 2024 — The American College of Cardiology (ACC) has announced its release of the ACC Remote Patient Management ...
July 24, 2024 — After a rigorous investigation procedure by the Food and Drug Administration, FibriCheck is now FDA ...

In a room of 20 people, it’s likely that about 10 of them, or half, will presently have some form of cardiovascular ...
Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...
June 26, 2024 — Anumana, a leading AI-driven health technology company and portfolio company of nference, and InfoBionic ...
May 10, 2024 — With cardiovascular disease being a leading cause of mortality worldwide, Vivalink, a leading provider of ...
This past spring, electrophysiology experts issued new recommendations for remote monitoring of cardiovascular ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
November 9, 2023 — According to a recent survey of healthcare providers (HCPs), 93 percent of clinicians are currently ...
August 7, 2023 — Movano Health, a purpose-driven healthcare solutions company at the intersection of medical and ...
August 7, 2023 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson M ...






 September 25, 2025
September 25, 2025