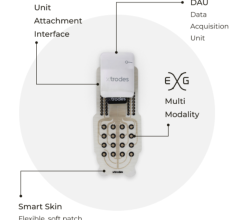
OTC ECG devices can help detect abnormal heart rhythms in the general population, and this technology will continue to improve.
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness product to enable wearers to get an electrocardiogram (ECG) from their wristwatch. The device monitors the user’s heart rate and rhythm, sending notifications if abnormal findings are detected. The Food and Drug Administration (FDA) cleared the product as an “over-the-counter” (OTC) Class II medical device, clearing the way for more brand entrants and follow-on next generation improvements, with greater opportunity for research on their health impact and efficacy. While these platforms continue to evolve, input from manufacturer product labeling, medical professionals and associations agree that current OTC ECG wearables have performance limitations and are not intended for medical diagnostic decision-making.
That doesn’t stop patients from bringing OTC device data to their doctors, though. Cardiologist Mohammed Abuzahra, M.D., said that each month, one or two patients in his Pikesville and Baltimore, Maryland practices raise questions about the heart data seen on their devices. He expects this number to rise as the technology becomes more prevalent and affordable. In addition to watches, Abuzahra has also seen ECG information gleaned from smartphone cases with a single ECG lead, where the data is captured when the patient puts a finger or fingers on the device.
Using wearables for health purposes is gaining popularity in the United States. A 2018 national survey showed that 49% of consumers want devices to monitor their heart health. The survey also showed 63% of respondents owned a wearable device currently or in the past, compared to 42% just two years earlier. Consumer demographics, underlying motivation for OTC health device adoption and long-term wear compliance implications are being more deeply studied.
“Some OTC devices have a fairly good track record of detecting silent but serious treatable cardiac arrhythmias, which are not a rare occurrence,” said Abuzahra. If the patient shows evidence of a suspected clinically actionable arrhythmia, given that most heart arrhythmias are inconsequential and benign, the doctor can use this as a data point to discuss potential further workup.
In the U.S., Atrial Fibrillation (AFib) is the most common serious cardiac arrhythmia that increases in incidence with age, and typically requires treatment and/or monitoring. AFib affects an estimated 2.7 to 6.1 million people, can occur without symptoms and increases a person’s risk of a life-changing stroke, heart failure and death. Detection of silent Afib in particular, could help physicians and their patients get an earlier confirmed diagnosis. With less labeled accuracy compared to prescriptive ECG devices, using an OTC ECG device could increase the potential for false positives and wasteful use of healthcare resources. Conversely, OTC ECG device false negatives could give patients a false sense of security, even if they experience other symptoms of an abnormal heart rhythm.
“Over the counter ECG technology is a “double-edged sword,” commented Abuzahra. “The devices can be helpful, but too much information can make patients anxious if they don’t understand how to interpret it, or don’t know when the device isn’t working.” A heart rate decrease to 40 beats per minute on the treadmill, for example, may be due to a technical issue, such as the electrode not making good contact with the skin.
“The patient’s full clinical history must be considered, and additional diagnostic testing completed before making a diagnosis and offering treatment, if needed,” concluded Abuzahra.
In spite of these concerns, Abuzahra is a fan of the technology. He’s recommended it for his patients who have experience working in the medical field, with some knowledge of heart arrhythmias. The devices can provide self-directed feedback for someone with an arrhythmia who wants to gauge whether their medication is working. But should all consumers use an ECG smartwatch or phone? Currently, Abuzahra steers patients without a medical background, and those who might get overly anxious about the results, away from wearable heart tracking devices.
Validating OTC wearable heart monitors
A Stanford University study followed 419,297 self-enrolled participants tracking ECG rhythms on Apple Watch and iPhone. During the nine-month study, almost 2,100 (0.5%) received notification of an irregular pulse – defined as the sensor detecting five out of six repeat episodes in 48 hours. These participants were instructed to call the study physician for a video consultation. Doctors recommended that 658 of those participants wear a heart monitor patch for a week, with 450 (68%) completing the task.
Of this latter group, the ECG readings found that 34% of them experienced atrial fibrillation episodes. In 84% of the cases where the participant wore the patch and watch concurrently, the ECG results matched. While promising, this level of accuracy does not meet the standards of ambulatory ECG devices used and prescribed by healthcare professionals.
Accuracy is rising in the devices, said Dr. Abuzahra, with the software and recording algorithms improving. That said, unlike wearable medical ECG devices designed to meet the stringent performance and testing standards required to be labeled for prescription-use, many OTC ECG devices are constrained by data-processing know-how and other technology limitations. Battery power, product size, form factor, consumer use design and not the least, consumer cost also come into play.
Prescription ECG Wearables Set the Standard for Medical Diagnosis
Prescription medical devices for heart rate and rhythm monitoring differ in that they rely on one or more “leads” or views of the heart and are typically attached to the chest wall. “This reduces motion artifact and provides more detailed information about cardiac electrical activity needed for medical diagnosis, “ said Robert Odell, President and Chief Operating Officer of Cardiac Insight, a digital health and ECG algorithm technology company based in Bellevue Washington.
As one example of the newer long-term continuous ECG recorders that record and report all cardiac electrical activity from 7 to 14 days, Cardiac Insight has developed and markets the Cardea SOLO system, a wearable ECG sensor and automated in-office cardiac arrhythmia analysis system prescribed by cardiologists, internal medicine, primary care and other medical professionals.
“OTC ECG devices are already helping detect abnormal heart rhythms in the general population and will continue to improve. They are not intended to replace prescription ECG devices or the care of your physician, however,” said Odell.
“Surveillance heart monitoring of those at risk is helpful as our population ages and acquires certain chronic conditions,” continued Odell, “but this also requires proof of consumer adoption and wear-time compliance. Someone periodically spot-checking their heart rhythm with an OTC ECG device may find an issue, but given that a significant percent (25-40%) of arrhythmias are silent, the diagnostic yield will be low. Perhaps in 10 or 20 years, with unlimited cloud storage and continuous monitoring, these OTC devices may be more helpful for population health,” said Odell.
One positive outcome from the many new OTC ECG devices is that they have raised awareness of the importance of the ECG as a diagnostic tool, said Odell.
Related Wearable ECG Monitoring Content:
Moving Beyond Holter ECG: The Rise in Next Generation Technology
How Advances in Wearable Cardiac Monitors Improve the Patient and Clinician Experience
Cardea Solo Wearable ECG Collects High-altitude Cardiac Data on Denali Expedition
Cardiac Insight Partners With VivoSense for Cardiovascular Research
Wearable Cardiac Monitors Are Effective for Tracking Atrial Fibrillation Following Ablation
As Interpretation Criteria Evolve, False Positive Athlete ECG Screening Rates Can Decrease


 January 14, 2026
January 14, 2026