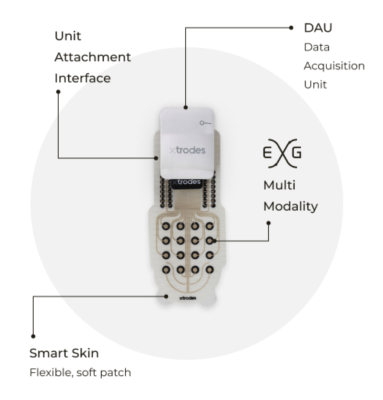
February 20, 2024 — X-trodes, a bio-convergence company bringing wireless monitoring solutions to the home environment, today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for X-trodes' Smart Skin solution (marketed as X-trodes System M), a new wireless wearable technologyfor advanced electrophysiological monitoring.
X-trodes’ Smart Skin is comprised of customizable dry-printed multi-modal electrode patches. It monitors a wide range of biopotential signals from anywhere on the body to acquire EEG (brain activity), EKG/ECG (cardiac monitoring), EOG (eye movement), and EMG (muscle activity) data. The discreet patches are easy to apply and comfortable to wear, conforming to the body without requiring gels, wires or uncomfortable solutions.
The FDA clearance follows successful completion of an extensive scientific assessment that evaluated the accuracy and consistency of X-trodes’ system. The study showed the performance of the X-trodes technology to be equivalent to that of FDA-cleared clinical electrophysiology devices in measuring EEG, EOG, EMG, and EKG/ECG signals.
“The X-trodes system is the next generation of wearable and fully wireless solutions, enabling clinicians and researchers to unleash the full potential of medical-grade electrophysiological monitoring,” said Ziv Peremen, PhD, CEO of X-trodes. “Gaining FDA clearance affirms the value of this technology and its potential to improve health and wellness through access to real-time electrophysiological data. It will further shorten the path to commercialization for a range of clinical use cases.”
Smart Skin is already available to researchers seeking unprecedented accuracy when measuring electrophysiological signals outside of laboratory settings. Having achieved FDA 510(k) clearance, X-trodes will pursue further validations for a wide range of clinical use cases, with an initial focus on the cardiovascular and sleep monitoring markets.
“Despite an abundance of legacy and new medical device companies entering the market, there is still no single configurable remote monitoring system capable of supporting neurology and cardiology departments in providing the best possible patient experience and outcomes," said Dr. Deganit Barak Shinar, VP of clinical affairs at X-trodes. “Our medical-grade multi-modal solution, which can be deployed across clinical departments and outpatient settings, including the home, has the potential to significantly improve the provision of care.”
Electrophysiological monitoring has traditionally been restricted to clinics, requiring cumbersome hardware and controlled environments to acquire highly sensitive physiological signals. X-trodes' Smart Skin encapsulates an entire monitoring lab into a discreet, flexible and completely wireless skin patch that provides continuous medical-grade monitoring in any environment that users may find themselves.
X-trodes' technology is backed by more than 15 years of research at Prof. Yael Hanein’s neuro engineering lab at Tel Aviv University and scientifically validated patents. Its efficacy has been supported by several peer-reviewed studies published in leading journals, including Nature Scientific Reports and the Journal of Neural Engineering.
For more information: htpps://xtrodes.com/


 January 15, 2026
January 15, 2026 









