Aug. 07, 2025 —- New research by European cybersecurity company Modat revealed more than 1.2 million internet-connected healthcare devices and systems are exposed and vulnerable to exploitation ...
Pharmaceuticals
This channel includes news and new technology innovations for cardiovascular pharmaceutics. This includes antiplatelet agents, anticoagulation drugs, INR testing, NOAC, OAC, IV administered drugs such as Heprin, and dual antiplatelet therapy.
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
Nov. 19, 2025 — Promising results from a pivotal Phase 3 clinical trial of an investigational heart-attack drug were ...
Sept. 25, 2025 — Kardigan has introduced the three late-stage clinical programs leading its robust pipeline, each ...
Sept. 23, 2025 — CeleCor Therapeutics’ multinational Phase 3 clinical trial of its investigational heart-attack drug ...
Sept. 2, 2025 —Corsera Health, Inc. has announced its launch with a mission to extend healthspan through cardiovascular ...
Aug. 07, 2025 —- New research by European cybersecurity company Modat revealed more than 1.2 million internet-connected ...
July 25, 2025 — Data in recent staffing surveys from the American Society of Radiologic Technologists show that vacancy ...
The American Heart Association recently announced that Dr. Christine E. Seidman, an internationally renowned physician ...
July 10, 2024 — Reviva Pharmaceuticals Holdings, Inc., a late-stage pharmaceutical company developing therapies that ...
June 26, 2024 — Semaglutide, a medication initially developed for type 2 diabetes and obesity, significantly improves ...
June 21, 2024 — Lexicon Pharmaceuticals, Inc. announced that the peer-reviewed Journal of the American College of ...
June 5, 2024 — Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX) today announced a new post-hoc analysis of clinical data ...
May 24, 2024 — Lexicon Pharmaceuticals, Inc. announced a new post-hoc analysis of clinical data showing that INPEFA ...
May 20, 2024 — Cardiologist Brendan Carry, MD, and a team of Danville, PA-based Geisinger Medical Center physicians have ...



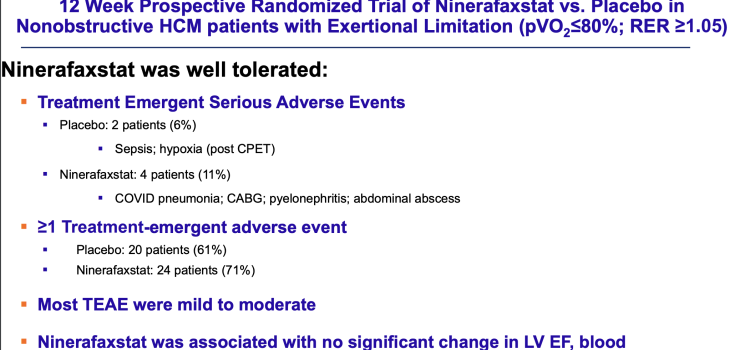
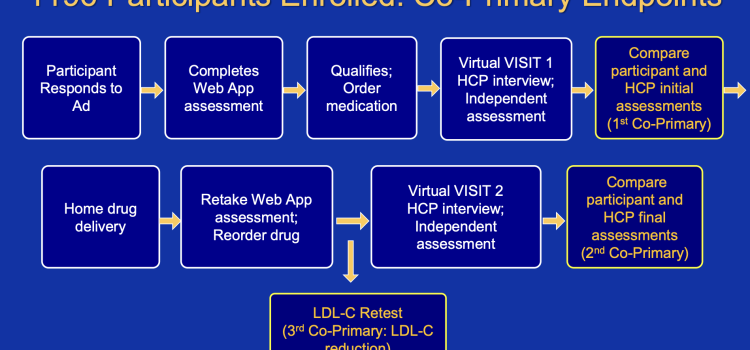

 January 28, 2026
January 28, 2026