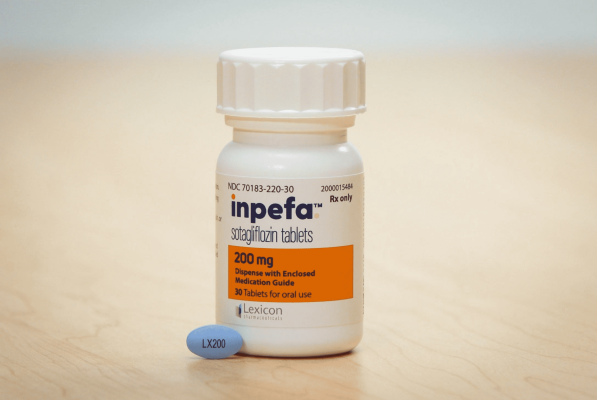
June 5, 2024 — Lexicon Pharmaceuticals, Inc. (Nasdaq: LXRX) today announced a new post-hoc analysis of clinical data showing that INPEFA® (sotagliflozin), a dual oral inhibitor of SGLT2 and SGLT1, reduced the risk of heart failure-related events across a diverse population of patients, including patients with preserved ejection fraction (HFpEF). Researchers noted that INPEFA appeared to be particularly effective in reducing the risk of heart failure events in patients with an obesity-related HFpEF phenotype. These findings, based on a pooled, patient-level analysis of data from the SOLOST-WHF and SCORED pivotal clinical trials, were presented today at the Annual Congress of the Heart Failure Association of the European Society of Cardiology (ESC) in Lisbon, Portugal.
Obesity and type 2 diabetes (T2D), along with a growing aging population, is contributing to the escalating prevalence of HFpEF. Recent data published in journals of the American College of Cardiology and the American Heart Association suggest that individuals with an obesity-related HFpEF phenotype represent a distinctive and clinically significant subgroup from those with standard HFpEF phenotype. This new analysis assessed the impact of obesity, along with sex and age, on the effects of INPEFA on the primary composite endpoint of cardiovascular (CV) death and heart failure (HF) events in patients with left ventricular ejection fraction (LVEF) ≥ 50%. Previously, SOLOIST-WHF and SCORED data demonstrated that INPEFA, a dual oral inhibitor of SGLT2 and SGLT1, is effective in reducing the risk of CV death and HF-related outcomes across the LVEF range.
Data from a total of 1,932 patients were included in the analysis (mean age: 69.9 years, mean BMI: 34.1 kg/m²; mean HbA1c:8.5%). In this population, 18.1% of patients experienced a primary endpoint event. Males and females demonstrated comparable event rates, 18.3% and 18.0% respectively; however, older age (< 65: 10.9% vs. ≥ 65years: 20.3%) and higher BMI (< 30 kg/m²: 16.6% vs. ≥ 30 kg/m²: 18.7%) were associated with an increased number of patients at risk for primary endpoint events.
Within the subgroup characterized by higher BMI, INPEFA therapy resulted in a favorable response for patients with BMI ≥ 30 kg/m² (p-value for interaction 0.038). Researchers also noted that both sex and age subgroups had a consistent response to INPEFA (p-value for interaction 0.818 and 0.393, respectively).
“This analysis underscores the importance of identifying patient risk factors such as age, sex, and obesity in patients with HFpEF and adds to the body of evidence differentiating INPEFA as a dual inhibitor of SGLT1 and SGLT2,” said Craig Granowitz, M.D., Ph.D., Lexicon’s senior vice president and chief medical officer. “Additionally, today’s data presentation further highlights the benefits of INPEFA in reducing the risk of heart failure-related events across a wide range of patients with HFpEF, including in patients with an obesity-related HFpEF phenotype.”
For more information: www.lexpharma.com


 August 29, 2025
August 29, 2025