Advances in coronary CT angiography (CCTA) over the past two decades have helped cardiologists detect heart disease in asymptomatic patients more accurately. Now, providers are using dedicated cardiac ...
Heart Failure
This heart failure channel offers news and new technology to treat heart failure. This includes for new innovations to treat congestive heart failure (CHF). The channel includes news on HFpEF and HFrEF. Heart failure occurs when the heart is no longer able to pump as much blood as the body requires. This can lead to enlargement of the heart because the muscle works harder to supply blood, but the pumping is ineffective. This may be due to defects in the myocardium, such as an infarct, or due to structural issues such as severe valve regurgitation. The disease is divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or a left ventricular assist device (LVAD).
Advances in coronary CT angiography (CCTA) over the past two decades have helped cardiologists detect heart disease in ...
Feb. 3, 2026 — Bristol Myers Squibb has launched "Change the Target. Change What’s Possible," an educational campaign ...
Jan. 28, 2026 — Corify Care has announced a major development in cardiac electrophysiology with the publication of its ...
New research shows superiority of Ultromics’ AI in predicting cardiac-related death As the use of artificial ...
Jan. 27, 2026 — Cytokinetics, Inc. has announced that Myqorzo (aficamten) is now available for prescription in 5 mg, 10 ...
Jan. 27, 2026 — A new national study reveals a stark disconnect between Americans’ desire for preventive cardiac ...
Jan. 20, 2026 — Abbott has received CE Mark in Europe for the TactiFlex Duo Ablation Catheter, Sensor Enabled to treat ...
Jan. 13, 2026 — A streamlined, nurse-led response for hospitalized patients experiencing an acute stroke at a Texas ...
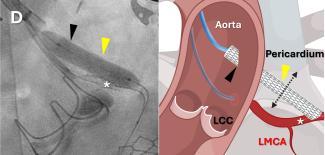
In a world first, a team of researchers at the National Institutes of Health (NIH) and Emory School of Medicine, Atlanta ...
Jan. 6, 2026 — Millions of Americans living with heart failure are not receiving medications that have been proven for ...
Dec. 16,2025 — The European Society of Cardiology (ESC) has welcomed the adoption of the Safe Hearts Plan, a landmark ...
Dec. 18, 2025 — Heartflow, Inc., a provider of AI technology for coronary artery disease (CAD), has announced the ...
Dec. 18, 2025 – Ventric Health, a medtech company enabling early detection of heart failure (HF) in a primary care ...
Dec.1, 2025 – MannKind Corp. has announced that the U.S. Food and Drug Administration (FDA) has accepted the sNDA ...
Nov. 10, 2025 —Genomics, a science-led techbio company, has today announced new research that suggests polygenic risk ...



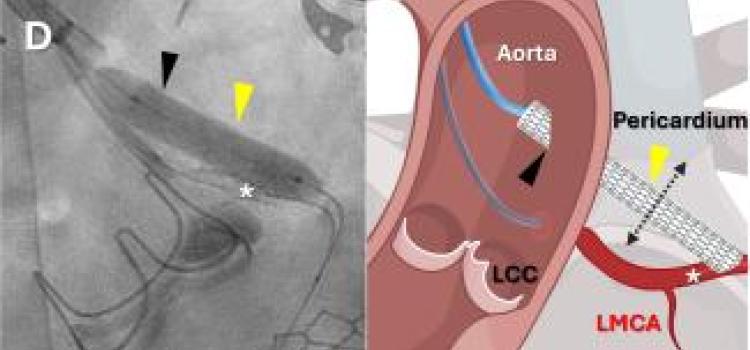
 February 06, 2026
February 06, 2026