April 29, 2024 — Women with heart disease are less often treated with cholesterol-lowering drugs than men, according to ...
Heart Failure
This heart failure channel offers news and new technology to treat heart failure. This includes for new innovations to treat congestive heart failure (CHF). The channel includes news on HFpEF and HFrEF. Heart failure occurs when the heart is no longer able to pump as much blood as the body requires. This can lead to enlargement of the heart because the muscle works harder to supply blood, but the pumping is ineffective. This may be due to defects in the myocardium, such as an infarct, or due to structural issues such as severe valve regurgitation. The disease is divided into four New York Heart Association (NYHA) classes. Stage IV heart failure is when the heart is completely failing and requires a heart transplant or a left ventricular assist device (LVAD).
April 25, 2024 —Heart-Valve-Surgery.com, a leading patient advocacy group for heart valve disease, with support from Med ...
April 23, 2024 — A recent study designed and implemented by investigators at Cedars-Sinai found that artificial ...
New research shows superiority of Ultromics’ AI in predicting cardiac-related death As the use of artificial ...
April 22, 2024 — At the annual American College of Cardiology conference (ACC.24) in Atlanta last week, RCE Technologies ...
April 22, 2024 — Corvia Medical, Inc, a company dedicated to transforming the treatment of heart failure, welcomes the ...
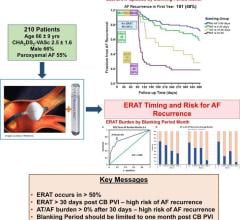
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period ...
April 18, 2024 — Bayer AG and Asklepios BioPharmaceutical, Inc., a gene therapy company wholly owned and independently ...
April 17, 2024 —CPR Therapeutics, Inc. (CPR-T), an early-stage medtech startup funded by the N.I.H and N.S.F to develop ...
April 16, 2024 — Each year more than 500,000 Americans undergo percutaneous coronary intervention, or PCI, a minimally ...
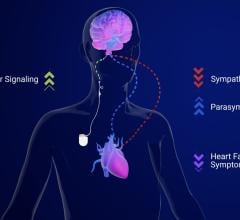
April 16, 2024 — CVRx, Inc., a commercial-stage medical device company, announced today the availability of additional ...
April 15, 2024 — The U.S. Food and Drug Administration (FDA) announced Abbott/Thoratec Corp. is recalling HeartMate II ...
April 12, 2024 — University of Virginia School of Medicine researchers have discovered a gene on the Y chromosome that ...
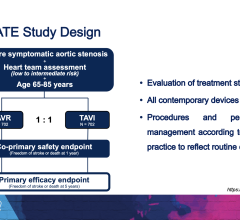
April 11, 2024 — Transcatheter aortic valve replacement (TAVR) was found to bring no increased risks and was associated ...
April 11, 2024 — People with a buildup of fatty atherosclerotic plaque in the heart’s arteries considered at risk of ...
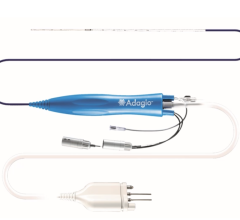
April 10, 2024 — A new technology using ultralow temperature cryoablation (ULTC) has eliminated clinical ventricular ...

 April 29, 2024
April 29, 2024