March 21, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the Instructions for Use for its Impella Left Sided Blood Pumps because the pump catheter may perforate ...
Left Atrial Appendage (LAA) Occluders
This channel includes news and new technology innovations about Left Atrial Appendage (LAA) Occluders. These close off the LAA in patients with atrial fibrillation to prevent the formation of stroke-causing clots in atrial fibrillation (AFib or AF) patients. LAA occlusion is often indicated for patients who do not tolerate anticoagulation therpy or have bleeding risks associated with use of that therapy.
April 4, 2024 — Osso VR, a leader in immersive procedural training, announces the release of a co-developed curriculum ...
March 21, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the Instructions for ...
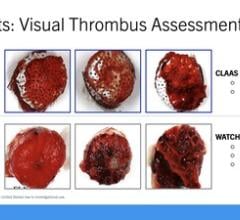
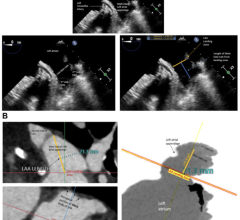
March 18, 2024 — Conformal Medical, Inc. announced that the CLAAS System was featured in a podium presentation at the Ca ...
January 8, 2024 — University Hospitals (UH) Harrington Heart & Vascular Institute recently became the first center in ...
October 25, 2023 — Findings from a trial led by Cleveland Clinic show that patients with atrial fibrillation undergoing ...
October 19, 2023 — Conformal Medical, Inc. announced today the one-year results from the company's CONFORMAL Early ...
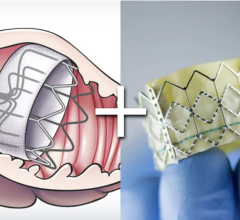
September 7, 2023 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration approval ...
July 27, 2023 — Abiomed is recalling the Impella Intravascular Left Sided Blood Pumps because the pump’s Instructions ...
July 26, 2023 — Abbott is recalling the Amplatzer Steerable Delivery Sheath due to an increased risk for air emboli to ...
May 25, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...
May 22, 2023 — Boston Scientific Corporation announced data supporting use of the company's key electrophysiology and ...
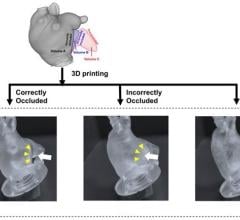
April 18, 2023 — Findings from an award-winning Scientific Online Poster presented during the 2023 ARRS Annual Meeting ...
March 28, 2023 — The Society for Cardiovascular Angiography and Interventions (SCAI) and the Heart Rhythm Society (HRS) ...
February 27, 2023 — Conformal Medical, Inc. announced today the results from the CONFORMAL Early Feasibility Study (EFS) ...




 April 04, 2024
April 04, 2024