March 18, 2024 — Conformal Medical, Inc. announced that the CLAAS System was featured in a podium presentation at the Cardiovascular Research Technologies (CRT) 2024 conference. Dr. William Gray, Professor of Medicine, Thomas Jefferson University, System Chief, Cardiovascular Diseases at Main Line Health, and Co-Director Lankenau Heart Institute presented "Comparative Acute Thrombogenicity of The CLAAS Foam Implant and Watchman FLX In An In Vitro Blood Loop Model" during the conference's Best Abstracts, March 10th.
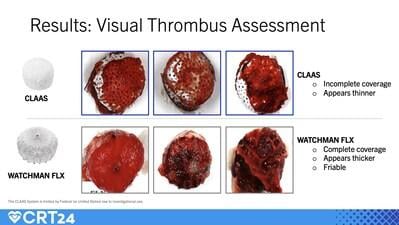
An in vitro study compared the relative thrombogenicity of the CLAAS implant to the Watchman FLX device. The CLAAS design features a foam cup with an embedded nitinol skeleton covered by a fluoropolymer (ePTFE) cover. The nitinol frame of the Watchman FLX is covered by polyethylene terephthalate (PET). Three of each device (n=3) were inserted into an acute radiolabeled in vitro blood loop system. After 90-120 minutes of exposure, the implants were visually assessed and radiolabels measured. When compared to the Watchman FLX, the CLASS device demonstrated:
- Incomplete coverage with seemingly thinner thrombus
- 44% lower platelet deposition
"These results are very encouraging, indicating the CLAAS implant appeared less thrombotic than the Watchman FLX in this in vitro blood loop model," commented Dr. Gray. "Device Related Thrombus (DRT) remains a concern in left atrial appendage closure and advances in device design may help mitigate this in the clinical setting. The ePTFE fluoropolymer component in the CLAAS device appears to be less thrombogenic which may reduce DRT; still, more studies are required to further validate these initial results."
Additionally, the study was recently published online in JACC: Cardiovascular Interventions.
Conformal Medical is actively enrolling patients in the CONFORM pivotal trial, evaluating the safety and efficacy of the CLAAS System compared to other commercially available LAAO devices. The prospective, multicenter, randomized controlled study will enroll approximately 1,600 patients.
For more information: www.conformalmedical.com


 January 05, 2026
January 05, 2026 









