May 27, 2025 — Despite scientific advances in cardiovascular care, people in living in rural areas and other communities with long-term economic or social challenges still face barriers to cutting-edge therapies such as gene editing, according to a 2020 American Heart Association presidential advisory.
To address gaps in care, the Association, devoted to changing the future of health for all, is using its Get With The Guidelines data to support patient populations that may be underrepresented or overlooked in clinical trials. Get With the Guidelines programs connect hospitals with current evidence-based guidelines and accurate measurement tools to improve care quality and industry practices.
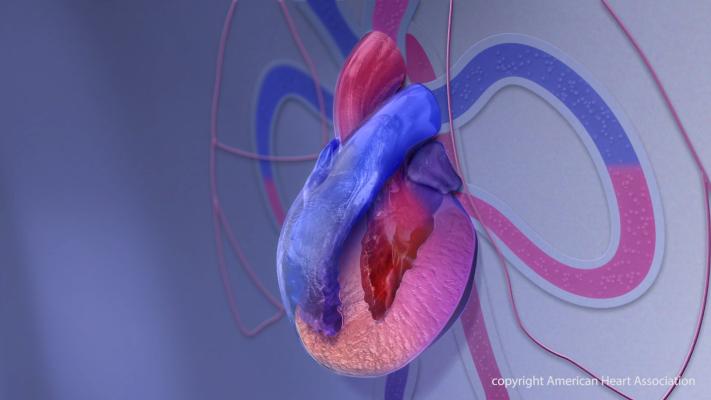
As part of this work, the Association today announced an initiative to improve education, outreach and access to clinical trials for gene editing therapies for transthyretin amyloid cardiomyopathy (ATTR-CM). ATTR-CM is a progressive and often underdiagnosed condition that disproportionately affects older adults and certain racial and ethnic groups.
In ATTR-CM, a misshapen form of the protein transthyretin builds up in the heart, preventing the left ventricle from relaxing and filling properly. Over time, this can impair the heart’s ability to pump blood, leading to heart failure.
This new effort builds on the American Heart Association’s ongoing commitment to improving diagnosis, care and outcomes for people with cardiomyopathy.
The objectives are to close knowledge gaps, raise awareness and improve access to early diagnosis and emerging treatments for cardiac amyloidosis — no matter where people live — through research, clinical education and public outreach. The nationwide initiative, conducted with financial support from Intellia Therapeutics, aims to elevate understanding of gene editing, advance research and support clinical trial opportunities among populations often excluded from research.
“Too many people remain unaware of or disconnected from lifesaving cardiovascular clinical trials,” said Michelle Kittleson, M.D., PhD, American Heart Association volunteer, professor of medicine at Cedars-Sinai and director of education, heart failure and transplantation at Smidt Heart Institute. “This effort is designed to help close that gap, ensuring that medical innovation is matched with education, trust and opportunity for all.”
The initiative includes a multi-pronged education and research initiative to assess current ATTR-CM awareness and develop new insights related to gene editing and cardiovascular disease. These observations will inform new educational materials, a series of national webinars and outreach strategies designed for patients, families and clinicians. The first webinar, Understanding Amyloidosis & Emerging Therapeutic Frontiers, will be held June 18, and will feature experts in cardiology and gene therapy.
Additional components of the initiative focus on enhancing patient identification for access to emerging therapies and clinical research opportunities. This includes activating a referral network of non-trial sites, supporting multidisciplinary provider education and developing tools that leverage clinical data to help identify potentially eligible participants.
More information is available at heart.org.


 January 05, 2026
January 05, 2026 









