Susan Cheng, M.D., MPH, director of the Institute for Research on Healthy Aging in the Department of Cardiology at Cedars-Sinai, moderated the Best Science in Cardiovascular Genetics and Genomics ...
Blood Testing
This channel includes news and new technology innovations for blood testing including platelet function monitors.
October 5, 2022 — New data presented at the Heart Failure Society of America (HFSA) Annual Scientific Meeting 2022, held ...

In a large-scale study of people from diverse ancestries, researchers narrowed down the number of genomic variants that ...
Susan Cheng, M.D., MPH, director of the Institute for Research on Healthy Aging in the Department of Cardiology at ...
July 15, 2021 — Using real-time deformability cytometry, researchers at the Max-Planck-Zentrum für Physik und Medizin in ...
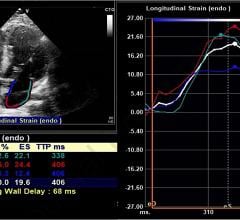
July 13, 2021 — Researchers at Johns Hopkins Medicine have shown that speckle-tracking strain echocardiograms may ...

The latest cardiology practice-changing scientific breakthrough, late-breaking study presentations have been announced ...
September 2, 2020 — An international, first-of-its-kind cardiology trial used personalized genetic testing to reduce by ...
March 28, 2020 — The TAILOR-PCI trial that used genetic testing to guide which antiplatelet medication was given to ...
December 18, 2019 — In their latest report, “Cardiovascular Disease 2020-2030: Trends, Technologies & Outlook” IDTechEx ...

November 5, 2019 — The U.S. Food and Drug Administration (FDA) is updating its November 2017 safety communication to ...
October 9, 2019 — Abbott recently announced that its Architect Stat High Sensitivity Troponin-I blood test has received ...
September 18, 2019 — Discharge of patients with suspected acute coronary syndromes under a 0- and 1-hour high ...

July 24, 2019 — Atrial fibrillation (AFib) is a common abnormal heart rhythm. It is treated either with medications or ...

Troponins are a family of proteins found in skeletal and heart (cardiac) muscle fibers that produce muscular contraction ...
March 19, 2019 — Prevencio Inc. announced data confirming the high accuracy of its artificial intelligence (AI)-driven ...

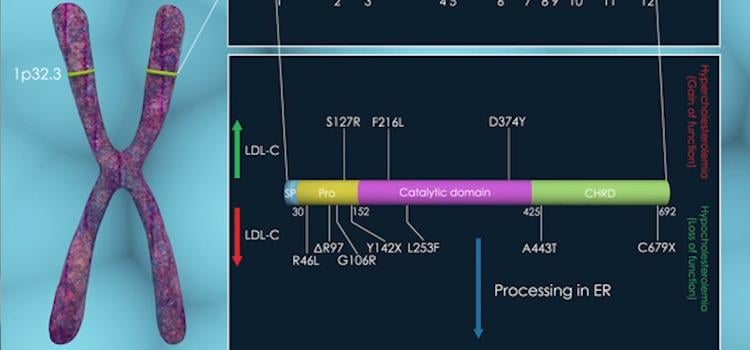




 October 05, 2022
October 05, 2022