By Dave Fornell, DAIC editor
The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) 2013 meeting brought many new insights about the latest cardiovascular technologies and treatment techniques. Among the hottest topics were transcatheter heart valve repairs, renal denervation to treat drug resistant hypertension, left atrial appendage (LAA) occluders to prevent stroke and eliminate warfarin therapy in atrial fibrillation patients, bifurcation stenting trials and techniques, new insights on anti-clotting agents, increasing use of ultrasound and intravascular imaging, new technologies for FFR lesion assessments, and the latest stent trial data.
I offer my editor’s choice for the most innovative new technologies presented in sessions and on the show floor in a video format.
For a video of the top meeting highlights, Herb Aronow, M.D., offers his views on some of the most interesting topics discussed in sessions.
The most exciting interventional device to enter the U.S. market a couple days prior to TCT was Abbott's MitraClip. We conducted a video interview with the lead author of the MitraClip FDA pivotal trial at TCT.
Here is a summary of some of the top news from TCT. You can click on any item to read the full story.
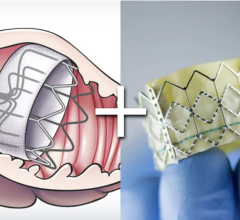
• CoreValve shows outcomes as good or better that Sapien
• CT-FFR shown to be comparable to invasive FFR
• Dedicated Tryton bifurcation stent did not meet primary endpoint
• Provisional vs. two-stent bifurcation therapy has similar outcomes
• Data promising for first FDA drug-eluting balloon trial
• Simplified, non-adenosine iFR compares favorably with standard FFR
• Hypothermia for STEMI safe, but did not reduce infarct size
• Thrombectomy does not improve outcomes in NSTEMI PCI
• MitraClip trial data leads to FDA approval — Video interview with lead author of study
• Bioresorbable polymer coated stent equal to traditional DES
• Boston Scientific’s Lotus TAVR valve meets primary endpoints in REPRISE II
• Pre-hospital bivalirudin for STEMI improves outcomes
• Data from two trials (ARCTIC-INTERRUPTION and OPTIMIZE) shows short-duration DAPT is feasible
• Genetic profiling for CYP2C19 antiplatelet agents improves outcomes
• Transradial access lowers bleeding risks in women
In addition to these news items, listed below are more top trial summaries and new product releases.



 April 04, 2024
April 04, 2024