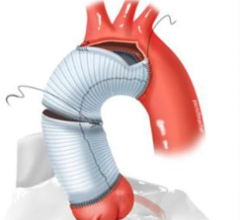
Dec. 26, 2025 — Edwards Lifesciences has announced the company’s SAPIEN M3 mitral valve replacement system, a transcatheter therapy using a transseptal approach, has received U.S. Food and Drug ...
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
Dec. 26, 2025 — Edwards Lifesciences has announced the company’s SAPIEN M3 mitral valve replacement system, a ...
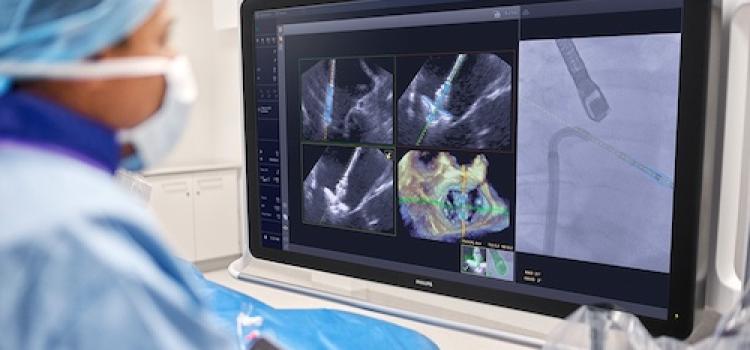
Nov. 17, 2025 — Royal Philips has introduced DeviceGuide, an AI-powered device tracking* solution that assists ...
Oct. 28, 2025 — People who underwent a minimally invasive procedure to have their heart’s aortic valve replaced had ...
Oct. 25, 2025 — Medtronic plc has announced the launch of the Stedi Extra Support guidewire, designed to enhance ...
Aug. 28, 2025 — Medtronic plc has announced it received U.S. Food and Drug Administration (FDA) approval for the ...
Aug. 18, 2025 — A new study published in The Annals of Thoracic Surgery, a journal from The Society of Thoracic Surgeons ...
Aug. 4, 2025 — Corcym announced that its Perceval Plus sutureless aortic heart valve was used in a first-ever robotic ...
July 10, 2025 — On July 2, 2025, Centers for Medicare & Medicaid Services (CMS) issued a National Coverage Determination ...
June 27, 2025 – Foldax Inc., a leader in the development of innovative polymer heart valves, has announced compelling ...
June 04, 2025 — HeartSciences Inc. has announced that the U.S. Food and Drug Administration has granted Breakthrough ...
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne ...
May 2, 2025 – New analysis from the EARLY TAVR trial showed patients between the age of 65 and 70 years old derived the ...
April 28, 2025 — The Society of Thoracic Surgeons (STS) has launched its latest surgical risk calculator designed for ...
March 30, 2025 — Medtronic has announced late-breaking data on five-year outcomes from the Evolut Low Risk Trial. Data ...




 December 24, 2025
December 24, 2025