May 15, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abbott is recalling the HeartMate 3 LVAS by ...
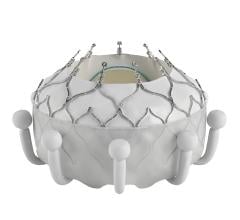
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
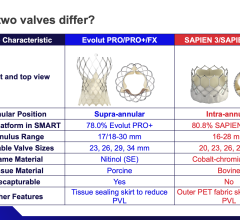
May 7, 2024 — Medtronic announced the release of important clinical outcomes in two leading transcatheter valve ...
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using Edward ...
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...
April 17, 2024 —CPR Therapeutics, Inc. (CPR-T), an early-stage medtech startup funded by the N.I.H and N.S.F to develop ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
April 9, 2024 — UC Davis Health cardiology team members are among the first in the country to treat patients with tricus ...
April 9, 2024 — Administering tranexamic acid (TxA), a drug used to reduce bleeding during heart surgery, topically ...
April 9, 2024 — People with a small aortic annulus, a part of the heart’s anatomy where the left ventricle meets the ...
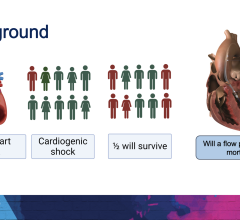
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
April 2, 2024 — Abbott announced that the U.S. Food and Drug Administration (FDA) approved the company's first-of-its ...
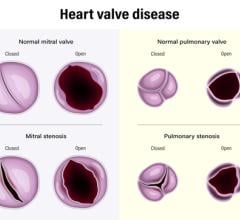
April 1, 2024 — Roughly 25,000 Americans die each year from valvular heart disease, but researchers from Rutgers Health ...
February 26, 2024 — Hackensack Meridian Jersey Shore University Medical Center and Hackensack University Medical Center ...

The DAIC team has learned of the passing of Alain Cribier, MD, FACC, heralded as the man who pioneered the first transca ...
February 2, 2024 — Edwards Lifesciences Corporation announced the company’s EVOQUE tricuspid valve replacement system is ...

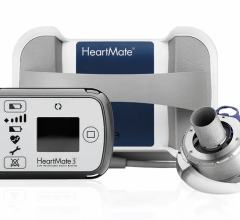
 May 15, 2024
May 15, 2024