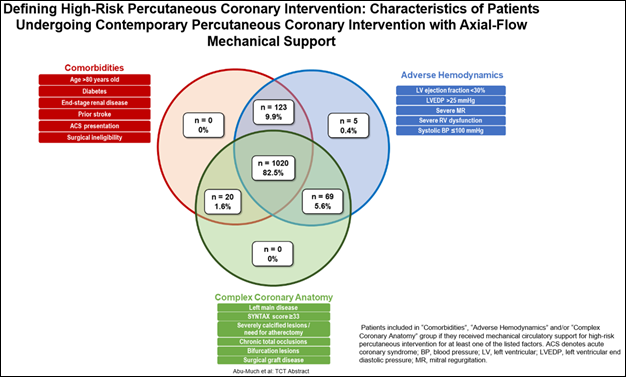
Figure 1: A PROTECT III study analysis demonstrates that 82.5% of Impella-supported HRPCI patients have features in all three high-risk factor domains – comorbidities, complex coronary anatomy and adverse hemodynamics.
October 24, 2023 — Abiomed, part of Johnson & Johnson MedTech[1], announced that novel data from seven research studies focused on optimizing outcomes for patients receiving Impella-supported high-risk percutaneous coronary intervention (HRPCI) will be presented at the 35th Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium taking place in San Francisco between Oct. 24 - 26.
As part of an academic collaboration coordinated by the Cardiovascular Research Foundation (CRF)[2], researchers conducted new analyses of data from the prospective, multicenter PROTECT III study, which includes the most robust contemporary data set of 1,237 Impella-supported HRPCI patients treated at 46 sites in the U.S. between March 2017- March 2020.
Impella Data Presentations
The seven studies include an analysis of the characteristics of PROTECT III patients that demonstrates how physicians are distinguishing which patients are high-risk and appropriate for Impella-supported HRPCI procedures. The data shows that 82.5 percent of patients have features in all three high-risk domains: comorbidities, complex coronary anatomy and adverse hemodynamics. It also demonstrates that 99.6 percent of patients have risk factors from two or more of the high-risk domains. (see figure 1)
Arsalan Abu-Much, MD, a cardiologist and postdoctoral fellow at CRF, will present this abstract (#TCT 216) on Thursday, Oct. 26 at 11:18 a.m. PDT at Station 6 – Emerging Clinical Science & Research, Hall C, Exhibition Level, Moscone South.
Additional Impella HRPCI abstracts being presented on Oct. 26 include (in order of presentation timing):
- TCT 143: Prognostic Implications of Anemia in Impella-Supported HRPCI: Insights From the cVAD PROTECT III Study
- TCT 145: Relationship Between Preprocedural Blood Pressure and Outcomes in Patients Undergoing Impella-Supported High-Risk PCI – Insights From the cVAD PROTECT III Study
- TCT 214: Right Ventricular Function in Patients Undergoing Impella-Assisted High-Risk Percutaneous Coronary Intervention: Insights From the cVAD PROTECT III Study
- TCT 241: Clinical Characteristics and Outcomes of Impella-Supported High-Risk PCI in Patients Turned Down for Coronary Artery Bypass Graft Surgery: Insights from the cVAD PROTECT III Study
- TCT 219: Performance of Existing Risk Models in Impella-Supported High-Risk Percutaneous Coronary Intervention
- TCT 240: A-SMART-EF: A Novel Score to Predict Mortality in Patients Undergoing Impella-Assisted Percutaneous Coronary Intervention
Daily Recap Webcast
- Abiomed will broadcast a daily summary of key TCT news from 5-5:30 p.m. PDT on Tuesday, Oct. 26, Wednesday Oct. 27 and Thursday, Oct. 28. Chuck Simonton, MD, Abiomed’s chief medical officer and Seth Bilazarian, MD, Abiomed’s vice president for professional education and medical communication, will host the daily recap shows. Visit https://www.heartrecovery.com/tct-2023 to tune in.
Abiomed at TCT 2023
- Abiomed’s World Connect TCT Symposium takes place on Tuesday, Oct. 24 between 11 a.m.–12 p.m. PDT on Level 2 in the Presentation Theater. The symposium, Safeguarding the Heart: Defining HRPCI, will be moderated by Bill O’Neill, MD, and include presentations from Aditya Bharadwaj, MD, Alexandra Lansky, MD, and Norman Mangner, MD. The symposium will be livestreamed.
- Abiomed’s Training Pavilion will take place on Tuesday, Oct. 24 between 2-3:30 p.m. and 4-5:30 p.m. PDT in Training Pavilion 1, Hall D, Exhibition Level, Moscone South. Attendees will hear from physician leaders on best practice techniques to optimize safety and efficacy using Impella for ventricular unloading. There will be stations for health care providers and/or attendees to get hands-on experience practicing access and closure, including image-guided access for both femoral and axillary approaches, and optimizing patients for ICU transfer. This training requires registration to attend.
- Abiomed’s booth (#915) will provide TCT attendees the opportunity to learn more about Abiomed’s Impella heart pump platform, including Impella RP, Impella RP Flex, Impella CP and Impella 5.5. Conference attendees are encouraged to stop by to learn more about Abiomed’s pipeline technology, including Impella ECP, engage in virtual reality training technology and get hands-on Impella experience with Advanced Impella trainers. Visit the booth Tuesday – Thursday, 8 a.m. – 6 p.m. PDT, located at Moscone South, Exhibition Level, Halls A-D.
For more information: www.abiomed.com
References:
[1] Abiomed, Inc. is part of Johnson & Johnson MedTech, which comprises the surgery, orthopedics, vision and interventional solutions businesses within Johnson & Johnson's MedTech segment.
[2] Abiomed and CRF have an academic collaboration supported by Abiomed and managed operationally by CRF.


 October 31, 2025
October 31, 2025 









