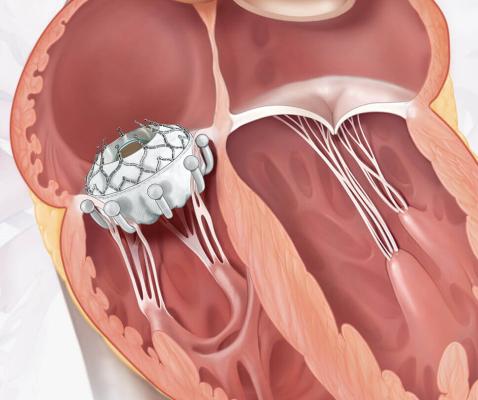
April 25, 2024 — Atlantic Health System’s Morristown Medical Center treated the first patient in New Jersey using Edwards Lifesciences EVOQUE tricuspid valve replacement, the first transcatheter therapy to receive U.S. Food and Drug Administration (FDA) approval for the treatment of tricuspid regurgitation (TR). The EVOQUE system is indicated for improving the health status in patients with symptomatic severe TR despite optimal medical therapy, and for whom tricuspid valve replacement is deemed appropriate by a heart care team. According to National Institutes of Health an estimated 1.6 million people in the U.S. have been diagnosed with TR.
“Until now, there was no available transcatheter treatment options for patients with tricuspid valve disease,” said Robert Kipperman, MD, interventional cardiologist and structural heart disease specialist at Gagnon Cardiovascular Institute’s Valve Center at Morristown Medical Center, part of Atlantic Health System. “We are thrilled to have a new transcatheter valve option which allows patients to feel significantly better by alleviating symptoms such as shortness of breath and can help improve the patients’ quality of life.”
About Atlantic Health System
Atlantic Health System is at the forefront of medicine, setting standards for quality health care in New Jersey, Pennsylvania and the New York metropolitan area. Powered by a workforce of 20,000 team members and 5,440 affiliated physicians dedicated to building healthier communities, Atlantic Health System serves more than half of the state of New Jersey including 14 counties and 7.5 million people. The not-for-profit system offers more than 550 sites of care, including its seven hospitals: Morristown Medical Center in Morristown, NJ, Overlook Medical Center in Summit, NJ, Newton Medical Center in Newton, NJ, Chilton Medical Center in Pompton Plains, NJ, Hackettstown Medical Center in Hackettstown, NJ, Goryeb Children’s Hospital in Morristown, NJ, Atlantic Rehabilitation Institute in Madison, NJ and through its partnership with CentraState Healthcare System in Freehold, NJ.
The system includes Atlantic Medical Group, part of a physician enterprise that makes up one of the largest multispecialty practices in New Jersey with more than 1,600 physicians and advance practice providers. Joined with Atlantic Accountable Care Organization and Optimus Healthcare Partners they form part of Atlantic Alliance, a Clinically Integrated Network of more than 2,500 health care providers throughout northern and central NJ.
Atlantic Health System provides care for the full continuum of health care needs through 24 urgent care centers, Atlantic Visiting Nurse and Atlantic Anywhere Virtual Visits. Facilitating the connection between these services on both land and air is the transportation fleet of Atlantic Mobile Health.Atlantic Health System leads the Healthcare Transformation Consortium, a partnership of six regional hospitals and health systems dedicated to improving access and affordability and is a founding member of the PIER Consortium – Partners in Innovation, Education, and Research – a streamlined clinical trial system that will expand access to groundbreaking research across five health systems in the region.
Atlantic Health System has a medical school affiliation with Thomas Jefferson University and is home to the regional campus of the Sidney Kimmel Medical College at Morristown and Overlook Medical Centers and is the official health care partner of the New York Jets.
For more information: www.atlantichealth.org
Related Content:
Edwards Evoque Transcatheter Tricuspid Valve Replacement System Receives CE Mark
Edwards Announces One-year Data on Transfemoral Transcatheter Tricuspid Valve Replacement
Six-Month Outcomes Positive for Transfemoral Tricuspid Valve in Tricuspid Regurgitation Patients


 January 15, 2026
January 15, 2026 









