Jan. 12, 2026 — HeartLung Corp. has announced U.S. Food and Drug Administration (FDA) clearance of AI-CVD, its AI-powered quantitative imaging platform, under 510(k) K252029.
With this clearance, AI-CVD becomes the most comprehensive FDA-cleared opportunistic screening platform available for CT imaging, enabling automated extraction of clinically relevant cardiovascular and systemic measurements from existing chest and abdominal CT scans — without additional imaging, radiation, contrast, or workflow disruption.
FDA-cleared AI-CVD can be applied to nearly 40 million CT scans performed annually in the United States, representing approximately half of all CT scans, and up to 80 million scans when including head and extremity imaging, transforming routine diagnostic imaging into a powerful, scalable engine for early detection and prevention.
A New FDA-Cleared Standard
AI-CVD is an opportunistic, AI-powered quantitative imaging tool that provides automated CT-derived anatomical and density-based measurements for clinician review.
Using AI-CVD quantitative imaging measurements and clinical evaluation, healthcare providers can investigate patients who are unaware of their risk of:
- Coronary heart disease
- Heart failure
- Atrial fibrillation
- Stroke
- Osteoporosis
- Liver steatosis
- Diabetes
- Other adverse health conditions that may warrant follow-up
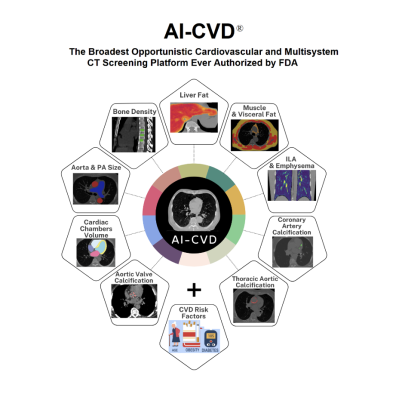
Ten FDA-Cleared Opportunistic Measurement Domains in a Single Platform
AI-CVD includes FDA-cleared modules for:
- Coronary artery calcium (CAC) scoring
- Aortic wall calcium
- Aortic valve calcium
- Mitral valve calcium
- Cardiac chamber volumetry
- Epicardial fat volumetry
- Aorta and pulmonary artery sizing
- Lung attenuation analysis
- Liver attenuation analysis
- Bone mineral density and muscle–fat composition
All volumetric measurements are adjusted for body surface area and reported in absolute values and population-based percentiles, referenced to the Multi-Ethnic Study of Atherosclerosis (MESA) and Framingham Heart Study (FHS).
“For decades, medicine has waited for patients to declare disease. AI-CVD allows us to find disease while it is still silent — using scans that already exist,” said Dr. Morteza Naghavi, founder and president of HeartLung Corp. “This FDA clearance represents a fundamental shift: CT is no longer just diagnostic imaging — it becomes a scalable, opportunistic prevention platform capable of identifying risk across the heart, lungs, bones, liver and metabolism in a single pass.”
By converting routine imaging into a multi-disease prevention platform, AI-CVD® addresses one of healthcare’s most persistent failures—missed opportunities to detect silent disease before irreversible events occur.
Advisory Board Perspectives
AI-CVD builds directly on the scientific foundations of modern cardiovascular imaging, as emphasized by members of HeartLung’s Scientific Advisory Board.
“Coronary calcium revealed long ago that atherosclerosis begins well before symptoms,” said Arthur Agatston, MD, Clinical Professor of Medicine, Florida International University, and developer of the Agatston Coronary Calcium Score. “AI-CVD extends that insight by enabling systematic identification of patients who are unaware of their cardiovascular risk—using CT scans that already exist.”
From the radiology perspective, the power of opportunistic screening lies in extracting more value from imaging already embedded in care pathways. “Modern CT contains far more clinically meaningful information than we traditionally extract,” said David Yankelevitz, MD, professor of Radiology, Icahn School of Medicine at Mount Sinai, and co-principal investigator of IELCAP. “AI-CVD allows clinicians to leverage routine CT scans responsibly and and we are now entering the new domain of comprehensive screening”
AI-CVD also reflects a growing recognition that cardiovascular disease is inseparable from broader systemic risk.
“Cardiovascular disease rarely exists in isolation,” said Zahi Fayad, PhD, Professor of Radiology and Medicine and Director of the BioMedical Engineering and Imaging Institute at Icahn School of Medicine at Mount Sinai. “By integrating quantitative measurements across the heart, vasculature, lungs, liver, bone and body composition, AI-CVD provides a scientifically grounded framework to identify individuals who may benefit from closer evaluation across multiple disease domains.”
The clinical impact of uncovering silent disease was underscored by translational experts. “Many of the most serious cardiovascular and metabolic conditions progress silently for years,” said Robert Kloner, MD, PhD, Director of the Cardiovascular Research Institute at Huntington Medical Research Institutes and Professor of Medicine at the Keck School of Medicine of USC. “This AI allows identification of structural abnormalities that the human eye may otherwise miss, hence helping to bring hidden risk into clinical focus, enabling informed decisions about further evaluation and prevention.”
Despite decades of progress, primary prevention today still relies largely on traditional risk factors and population-based scores, which frequently miss individuals with substantial subclinical disease. Opportunistic, CT-based quantitative imaging offers a long-overdue opportunity to modernize prevention by directly identifying structural and calcific disease burden rather than inferring risk indirectly.
““Primary prevention has long depended on risk factors and probability scores, yet we continue to see cardiovascular events in patients who were never identified as high risk. Quantitative CT findings—such as markedly elevated coronary calcium and enlargement of cardiac chambers, particularly the left atrium and ventricle—directly reveal underlying disease burden. AI-CVD® provides a scalable, FDA-cleared pathway to incorporate these objective phenotypes into preventive care, representing a necessary evolution of modern prevention.” — Nathan D. Wong, PhD, MPH, FACC, FAHA, FNLA, MASPC Professor of Medicine; Director, Heart Disease Prevention Program; Co-Director, Center for Global Cardiometabolic Health and Nutrition, University of California, Irvine; Past President, American Society for Preventive Cardiology
Transforming CT
Unlike traditional screening programs that require dedicated exams, referrals and reimbursement pathways, AI-CVD operates as an opportunistic add-on to CT scans already being performed for other clinical indications, including:
- Lung cancer screening CT
- Coronary calcium scans
- Diagnostic chest CT
- Coronary CT angiography (CCTA)
- CT pulmonary angiography (CTPA)
- Abdominal and pelvic CT
By converting routine imaging into a multi-disease prevention platform, AI-CVD® addresses one of healthcare’s most persistent failures—missed opportunities to detect silent disease before irreversible events occur.
A Regulatory Milestone
With this clearance, HeartLung now holds FDA authorization across ten opportunistic screening indications within a single AI platform, positioning AI-CVD as a foundational technology for population-scale preventive imaging.
As healthcare systems increasingly shift toward early detection, value-based care, and cost containment, AI-CVD® provides a regulatory-cleared pathway to extract far greater clinical value from imaging that already exists — without burdening patients or providers.
For more information, visit www.heartlung.ai


 January 28, 2026
January 28, 2026 









