March 29, 2022 – Artio Medical, Inc., a medical device company developing innovative products for the peripheral vascular, neurovascular, and cardiology markets, has received US Food and Drug ...
Vascular Closure Devices
This channel includes news and new technology innovations for vascular closure devices used to rapidly seal and achive hemostatsis at vascular access sites in interventional cardiology procedures.
Oct. 7, 2025 — Nobles Medical Technology II, a provider of cardiovascular closure solutions, has announced that the U.S ...
May 8, 2024 — 4C Medical Technologies, Inc. ("4C Medical"), a medical device company dedicated to advancing minimally ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
September 16, 2022 — Teleflex Incorporated, a leading global provider of medical technologies, announced that Dr. Magnus ...
September 15, 2022 — Cardiac Implants LLC has announced the successful initial deployment of its Annuloplasty ring with ...
March 29, 2022 – Artio Medical, Inc., a medical device company developing innovative products for the peripheral ...
November 9, 2021 — Results from the largest randomized trial available comparing different closure device strategies ...
July 15, 2021 — Vivasure Medical announced its development program for PerQseal Blue, a sutureless and fully ...
Arnold Seto, M.D., MPA, FSCAI, chief of cardiology, Long Beach Veterans Affairs Medical Center and director ...
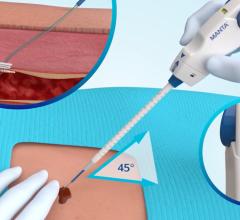
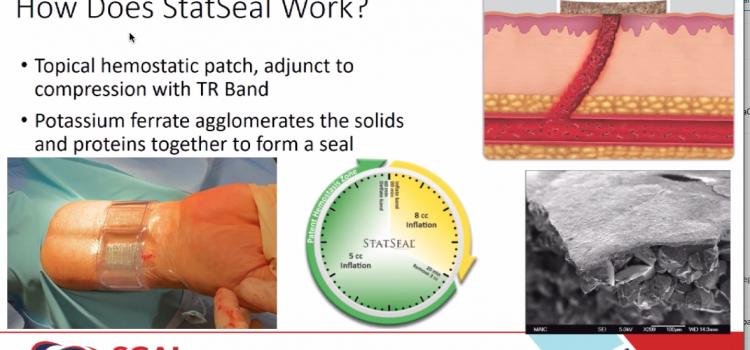
April 28, 2021 — A new study reveals the use of a potassium ferrate hemostatic patch (PFHP) reduces the time to ...
April 21, 2021 — EnsiteVascular announced it received its second U.S. Food and Drug Administration (FDA) market ...
August 17, 2020 — Veryan Medical announced it will support Vasorum in the commercialization of the Celt atrial closure ...
December 30, 2019 — Merit Medical Systems Inc., a leading manufacturer of proprietary devices used primarily in ...
November 6, 2019 — Saranas Inc. announced completion of the first U.S. commercial case using its Early Bird Bleed ...
Ashish Pershad, M.D., chief of interventional cardiology, Banner University Medical Center, Phoenix, explains the trend ...

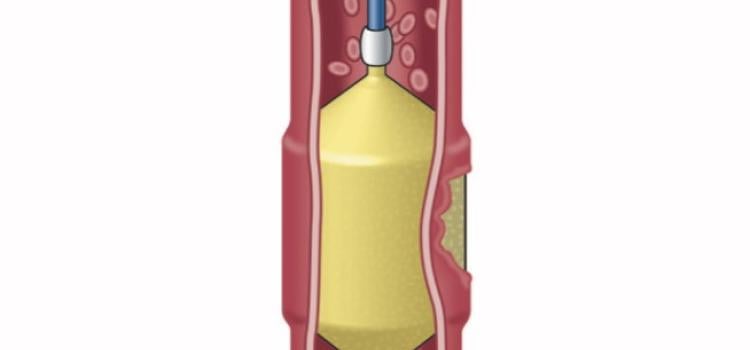
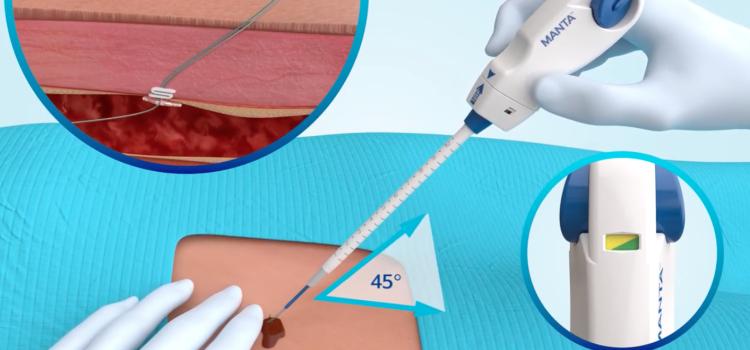
 October 07, 2025
October 07, 2025