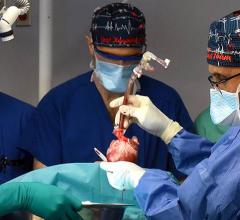
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial Heart (TAH), announced that its device has received Breakthrough Device ...
Artificial Heart
This channel includes news and new technology innovations for total artificial hearts (TAH). These technologies are used in cardiovascular surgery to temporarily or permanently replace the heart in advanced heart failure patients.
May 30, 2025 — BiVACOR, a clinical-stage medical device company developing the world’s first titanium Total Artificial ...
Jan. 29, 2025 – Scandinavian Real Heart AB (recently aannounced that its total artificial heart, Realheart TAH, has been ...
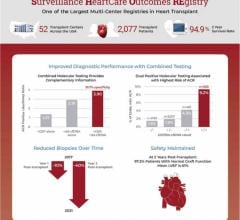
May 31, 2024 — CareDx, a leading precision medicine company focused on the discovery, development, and commercialization ...

HeartFlow is offering a free webinar based on a recent study that measured the ability of the of its roadmap ...
November 20, 2023 — Student engineers from the University of Bath are on top of the world after winning an international ...
November 10, 2023 — BiVACOR, a clinical-stage medical device company developing the Total Artificial Heart (TAH), has ...
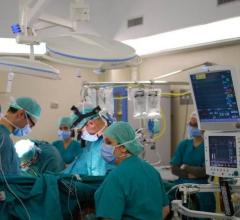
September 25, 2023 — After world’s first successful transplant in 2022, also performed at the University of Maryland ...
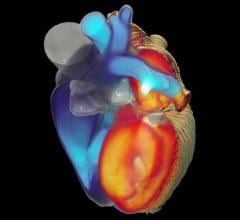
September 19, 2023 — A mathematical and computational model of the human heart entirely developed at Politecnico di ...
August 9, 2023 — A team of researchers, led by the Hebrew University of Jerusalem (HU), has developed a tiny human heart ...

The U.S. organ donation system has long been under scrutiny, with questions about fairness in organ allocation and ...

During the AIMed 2023 Summit, a panel of experts shared their personal perspective on what’s happening in artificial ...
July 3, 2023 — Nearly one-third of patients with an implanted device to prevent sudden death have anxiety in the first ...

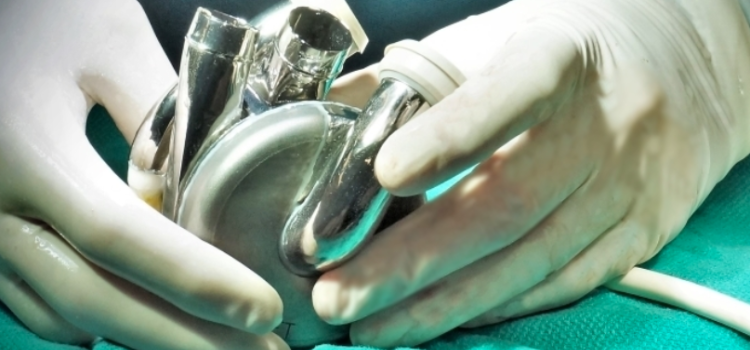
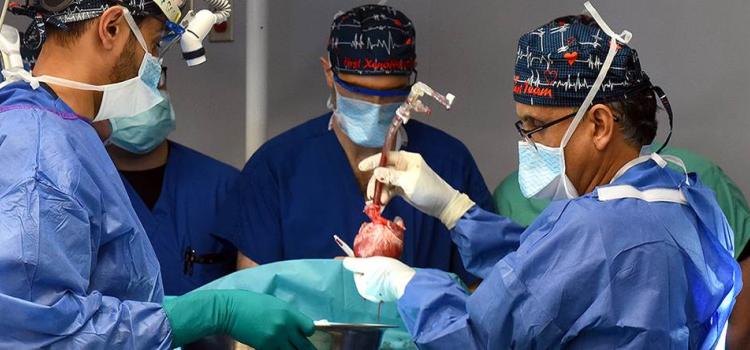



 May 30, 2025
May 30, 2025