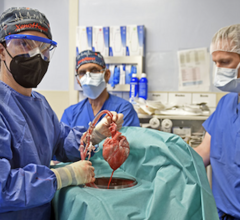
June 30, 2023 — A new study published in The Lancet has revealed the most extensive analysis to date on what led to the ...
Artificial Heart
This channel includes news and new technology innovations for total artificial hearts (TAH). These technologies are used in cardiovascular surgery to temporarily or permanently replace the heart in advanced heart failure patients.
June 28, 2023 — Liver disease, the UK’s third leading cause of premature death, poses a significantly greater threat to ...
June 2, 2023 — Dr. Philip Cardiff, Associate Professor at University College Dublin's School of Mechanical and Materials ...

It's been a fruitful month for DIcardiology.com! Here's a look at what DAIC viewers found to be most interesting during ...
May 1, 2023 — Picard Medical, Inc. (“Picard Medical”), the parent company of SynCardia Systems, LLC (“SynCardia”), a ...
April 26, 2023 — In the majority of cases, graft failure after heart transplantation is attributable to abnormalities ...
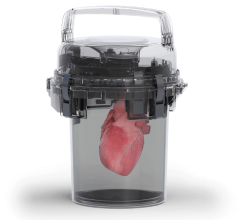
April 6, 2023 — Paragonix Technologies, Inc., a leading organ transplant company, announces new research from a multi ...
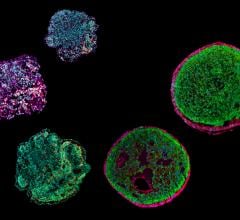
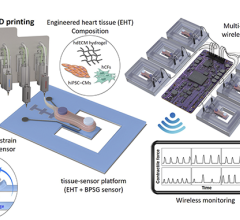
April 4, 2023 — A team at the Technical University of Munich (TUM) has induced stem cells to emulate the development of ...
March 29, 2023 — Cardiotoxicity is a clinical condition that arises from using pharmaceutical agents such as antibiotics ...
March 17, 2023 — More donated hearts could be suitable for transplantation if they are kept functioning within the body ...
March 4, 2023 — GE HealthCare will present its latest technologies and innovations for use during the critical planning ...
February 17, 2023 — Allegheny Health Network’s (AHN) Cardiovascular Institute reached a significant milestone in cardiac ...
February 17, 2023 — Internationally recognized cardiologist in heart transplantation Jon Kobashigawa, MD, director of ...
January 13, 2023 — A change to Medicare policy surrounding heart transplant may lead to increased inequities in access ...
November 15, 2022 — Ten months after transplanting the first genetically-modified pig heart into a human patient ...

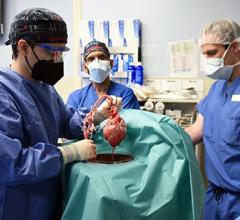
 June 30, 2023
June 30, 2023